Assessing the Effect of Smokeless Tobacco Consumption on Oral Microbiome in Healthy and Oral Cancer Patients
- PMID: 35433507
- PMCID: PMC9009303
- DOI: 10.3389/fcimb.2022.841465
Assessing the Effect of Smokeless Tobacco Consumption on Oral Microbiome in Healthy and Oral Cancer Patients
Abstract
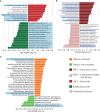
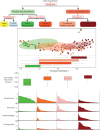
Oral cancer is a globally widespread cancer that features among the three most prevalent cancers in India. The risk of oral cancer is elevated by factors such as tobacco consumption, betel-quid chewing, excessive alcohol consumption, unhygienic oral condition, sustained viral infections, and also due to dysbiosis in microbiome composition of the oral cavity. Here, we performed an oral microbiome study of healthy and oral cancer patients to decipher the microbial dysbiosis due to the consumption of smokeless-tobacco-based products and also revealed the tobacco-associated microbiome. The analysis of 196 oral microbiome samples from three different oral sites of 32 healthy and 34 oral squamous cell carcinoma (OSCC) patients indicated health status, site of sampling, and smokeless tobacco consumption as significant covariates associated with oral microbiome composition. Significant similarity in oral microbiome composition of smokeless-tobacco-consuming healthy samples and OSCC samples inferred the possible role of smokeless tobacco consumption in increasing inflammation-associated species in oral microbiome. Significantly higher abundance of Streptococcus was found to adequately discriminate smokeless-tobacco-non-consuming healthy samples from smokeless-tobacco-consuming healthy samples and contralateral healthy site of OSCC samples from the tumor site of OSCC samples. Comparative analysis of oral microbiome from another OSCC cohort also confirmed Streptococcus as a potential marker for healthy oral microbiome. Gram-negative microbial genera such as Prevotella, Capnocytophaga, and Fusobacterium were found to be differentially abundant in OSCC-associated microbiomes and can be considered as potential microbiome marker genera for oral cancer. Association with lipopolysaccharide (LPS) biosynthesis pathway further confirms the differential abundance of Gram-negative marker genera in OSCC microbiomes.
Keywords: dignostic biomarker; microbiome & dysbiosis; oral microbiome shift; oral squamos cell carcinoma; tobacco consumption.
Copyright © 2022 Saxena, Prasoodanan P K, Gupta, Gupta, Waiker, Samaiya, Sharma and Sharma.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Al-Hebshi N. N., Nasher A. T., Maryoud M. Y., Homeida H. E., Chen T., Idris A. M., et al. . (2017). Inflammatory Bacteriome Featuring Fusobacterium Nucleatum and Pseudomonas Aeruginosa Identified in Association With Oral Squamous Cell Carcinoma. Sci. Rep. 71 (7), 1–10. doi: 10.1038/s41598-017-02079-3 - DOI - PMC - PubMed
-
- Amarasinghe A. A. H. K., Usgodaarachchi U. S., Johnson N. W., Warnakulasuriya S. (2018). High Prevalence of Lifestyle Factors Attributable for Oral Cancer, and of Oral Potentially Malignant Disorders in Rural Sri Lanka. Asian Pac. J. Cancer Prev. 19, 2485. doi: 10.22034/APJCP.2018.19.9.2485 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

