Multicomponent Solids of Niflumic and Mefenamic Acids Based on Acid-Pyridine Synthon
- PMID: 35433637
- PMCID: PMC9009247
- DOI: 10.3389/fchem.2022.729608
Multicomponent Solids of Niflumic and Mefenamic Acids Based on Acid-Pyridine Synthon
Abstract
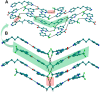
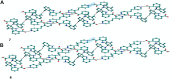
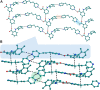
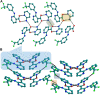
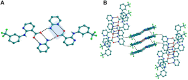
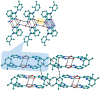
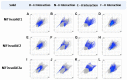
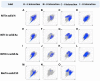
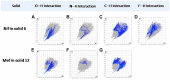
The present study discusses comparative structural features of fourteen multicomponent solids of two non-steroidal anti-inflammatory drugs, Niflumic and Mefenamic acids, with amine and pyridine-based coformers. All the solids were structurally characterized through PXRD, SCXRD, DSC, and the monophasic nature of some of the solids was established through Rietveld refinement. The solid forms include salt, cocrystal, hydrate, and solvate. Except for two, all the solids reported here showed relatively higher solubility compared to the acids. The difference in pKa and similarity in structural features of both the molecules enabled us to study the effect of ΔpKa on crystallization outcome systematically. The structures of all the solids are described through acid-pyridine synthon perspective.
Keywords: acid-pyridine synthon; cocrystallization; hirshfeld surface analysis; intermolecular interactions; ΔpKa rule.
Copyright © 2022 Kumar, Goswami, Balendra, Tewari and Ramanan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures










References
-
- Bhattacharya B., Das S., Lal G., Soni S. R., Ghosh A., Reddy C. M., et al. (2020). Screening, crystal Structures and Solubility Studies of a Series of Multidrug Salt Hydrates and Cocrystals of Fenamic Acids with Trimethoprim and Sulfamethazine. J. Mol. Struct. 1199, 127028. 10.1016/j.molstruc.2019.127028 - DOI
-
- Bodnár K., Hudson S. P., Rasmuson Å. C. (2017). Stepwise Use of Additives for Improved Control over Formation and Stability of Mefenamic Acid Nanocrystals Produced by Antisolvent Precipitation. Cryst. Growth Des. 17, 454–466. 10.1021/acs.cgd.6b01256 - DOI
-
- Bruker Analytical X-ray Systems (2000). SMART: Bruker Molecular Analysis Research Tool. Madison, WI: Bruker AXS.
-
- Bruker Analytical X-ray Systems, SAINT-NT (2001). Bruker Analytical X-ray Systems. SAINT + NT, Version 6.04. Madison, WI. Bruker AXS.
LinkOut - more resources
Full Text Sources

