Incidence Trend and Competing Risk Analysis of Patients With Intrahepatic Cholangiocarcinoma: A Population-Based Study
- PMID: 35433765
- PMCID: PMC9005886
- DOI: 10.3389/fmed.2022.846276
Incidence Trend and Competing Risk Analysis of Patients With Intrahepatic Cholangiocarcinoma: A Population-Based Study
Abstract
Background: Intrahepatic cholangiocarcinoma (ICCA) is a primary liver cancer characterized by rapid progression and poor prognosis. There are few effective tools for evaluating the prognosis of ICCA patients, and the use of liver transplantation (LT) of the treatment for ICCA is still controversial.
Methods: We analyzed ICCA incidence data and clinicopathological data from the Surveillance, Epidemiology, and End Results database. Prognostic predictors were identified by univariate and multivariate Cox regression analyses and then used to establish a nomogram. The prediction performance of the nomogram was evaluated with receiver operating characteristic (ROC) curves, calibration plots and decision curve analysis (DCA) plots. Propensity score matching (PSM) was used to balance the baseline data of patients undergoing LT and other operations, and then, univariate Cox regression analysis was used to evaluate the therapeutic value of LT for ICCA.
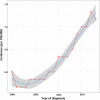
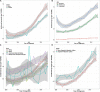
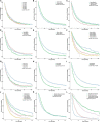
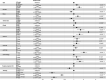
Results: The incidence of ICCA increased significantly, from 0.6 per 100,000 in 2,000 to 1.3 per 100,000 in 2018. The median overall survival (OS) of the patients was 13 months, and the 1-, 3-, and 5-year OS rates were 51.40, 22.14, and 13.79%, respectively. Cox regression analysis showed that age under 60 years old, female, tumor size ≤ 50 mm, better differentiation, smaller range of tumor invasion, lack of distant metastasis, regional lymph node surgery and treatment were associated with a better prognosis. The ROC curves, calibration plots, and DCA plots showed that the nomogram had good discrimination and calibration power, as well as clinical utility. After PSM, the univariate Cox regression analysis showed no significant difference in OS between patients treated with LT and patients treated with other operations.
Conclusion: The incidence of ICCA increased significantly. A nomogram with good predictive performance was developed to predict the OS of ICCA patients. LT might be considered as a potential option for some ICCA patients.
Keywords: intrahepatic cholangiocarcinoma; liver transplantation; nomogram; prognosis; risk factor.
Copyright © 2022 Xing, Tan, Yang and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures








References
LinkOut - more resources
Full Text Sources
Miscellaneous

