AAV-mediated BMP7 gene therapy counteracts insulin resistance and obesity
- PMID: 35434177
- PMCID: PMC8983313
- DOI: 10.1016/j.omtm.2022.03.007
AAV-mediated BMP7 gene therapy counteracts insulin resistance and obesity
Abstract
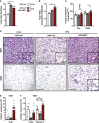
Type 2 diabetes, insulin resistance, and obesity are strongly associated and are a major health problem worldwide. Obesity largely results from a sustained imbalance between energy intake and expenditure. Therapeutic approaches targeting metabolic rate may counteract body weight gain and insulin resistance. Bone morphogenic protein 7 (BMP7) has proven to enhance energy expenditure by inducing non-shivering thermogenesis in short-term studies in mice treated with the recombinant protein or adenoviral vectors encoding BMP7. To achieve long-term BMP7 effects, the use of adeno-associated viral (AAV) vectors would provide sustained production of the protein after a single administration. Here, we demonstrated that treatment of high-fat-diet-fed mice and ob/ob mice with liver-directed AAV-BMP7 vectors enabled a long-lasting increase in circulating levels of this factor. This rise in BMP7 concentration induced browning of white adipose tissue (WAT) and activation of brown adipose tissue, which enhanced energy expenditure, and reversed WAT hypertrophy, hepatic steatosis, and WAT and liver inflammation, ultimately resulting in normalization of body weight and insulin resistance. This study highlights the potential of AAV-BMP7-mediated gene therapy for the treatment of insulin resistance, type 2 diabetes, and obesity.
Keywords: AAV; BMP7; gene therapy; insulin resistance; obesity; type 2 diabetes.
© 2022 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Duclos M. Osteoarthritis, obesity and type 2 diabetes: the weight of waist circumference. Ann. Phys. Rehabil. Med. 2016;59:157–160. - PubMed
-
- Van Gaal L.F., Mertens I.L., De Block C.E. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444:875–880. - PubMed
-
- Renehan A.G., Tyson M., Egger M., Heller R.F., Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. - PubMed
-
- Association A.D. Classification and diagnosis of diabetes: standards of medical care in Diabetesd2018. Diabetes Care. 2018;41:S13–S27. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

