ENO1 and Cancer
- PMID: 35434271
- PMCID: PMC8987341
- DOI: 10.1016/j.omto.2021.12.026
ENO1 and Cancer
Abstract
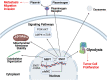
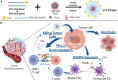
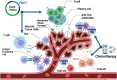
α-Enolase (ENO1), also known as 2-phospho-D-glycerate hydrolase, is a glycolytic enzyme that catalyzes the conversion of 2-phosphoglyceric acid to phosphoenolpyruvic acid during glycolysis. It is a multifunctional oncoprotein that is present both in cell surface and cytoplasm, contributing to hit seven out of ten "hallmarks of cancer." ENO1's glycolytic function deregulates cellular energetic, sustains tumor proliferation, and inhibits cancer cell apoptosis. Moreover, ENO1 evades growth suppressors and helps tumors to avoid immune destruction. Besides, ENO1 "moonlights" on the cell surface and acts as a plasminogen receptor, promoting cancer invasion and metastasis by inducing angiogenesis. Overexpression of ENO1 on a myriad of cancer types together with its localization on the tumor surface makes it a great prognostic and diagnostic cancer biomarker as well as an accessible oncotherapeutic target. This review summarizes the up-to-date knowledge about the relationship between ENO1 and cancer, examines ENO1's potential as a cancer biomarker, and discusses ENO1's role in novel onco-immunotherapeutic strategies.
Keywords: ENO1; cancer biomarker; glycolysis; hallmarks of cancer; oncotherapy; tumorigenesis.
© 2022 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

