Superionic Conduction in the Plastic Crystal Polymorph of Na4P2S6
- PMID: 35434367
- PMCID: PMC9008513
- DOI: 10.1021/acsenergylett.1c02815
Superionic Conduction in the Plastic Crystal Polymorph of Na4P2S6
Abstract
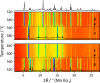
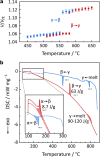
Sodium thiophosphates are promising materials for large-scale energy storage applications benefiting from high ionic conductivities and the geopolitical abundance of the elements. A representative of this class is Na4P2S6, which currently shows two known polymorphs-α and β. This work describes a third polymorph of Na4P2S6, γ, that forms above 580 °C, exhibits fast-ion conduction with low activation energy, and is mechanically soft. Based on high-temperature diffraction, pair distribution function analysis, thermal analysis, impedance spectroscopy, and ab initio molecular dynamics calculations, the γ-Na4P2S6 phase is identified to be a plastic crystal characterized by dynamic orientational disorder of the P2S6 4- anions translationally fixed on a body-centered cubic lattice. The prospect of stabilizing plastic crystals at operating temperatures of solid-state batteries, with benefits from their high ionic conductivities and mechanical properties, could have a strong impact in the field of solid-state battery research.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Wiberg N.Lehrbuch der Anorganischen Chemie; De Gruyter, 2008.
-
- Yang Y.; Song M.; Wu X.; Wu K. A review of the structural diversity of [PxSy]n– motifs and their potential application prospects in metal thiophosphates. J. Phys. D 2021, 54, 463002.10.1088/1361-6463/ac1538. - DOI
-
- Jansen M.; Henseler U. Synthesis, structure determination, and ionic conductivity of sodium tetrathiophosphate. J. Solid State Chem. 1992, 99, 110–119. 10.1016/0022-4596(92)90295-7. - DOI
-
- Hayashi A.; Noi K.; Tanibata N.; Nagao M.; Tatsumisago M. High sodium ion conductivity of glass–ceramic electrolytes with cubic Na3PS4. J. Power Sources 2014, 258, 420–423. 10.1016/j.jpowsour.2014.02.054. - DOI
LinkOut - more resources
Full Text Sources
