Tollip promotes hepatocellular carcinoma progression via PI3K/AKT pathway
- PMID: 35434373
- PMCID: PMC8976180
- DOI: 10.1515/med-2022-0453
Tollip promotes hepatocellular carcinoma progression via PI3K/AKT pathway
Erratum in
-
Erratum to "Tollip promotes hepatocellular carcinoma progression via PI3K/AKT pathway".Open Med (Wars). 2022 Jun 11;17(1):1065. doi: 10.1515/med-2022-0501. eCollection 2022. Open Med (Wars). 2022. PMID: 35799604 Free PMC article.
Abstract
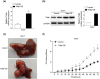
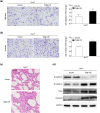
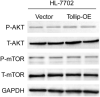
The activation of signaling pathways induced by Toll-like receptor (TLR) has been demonstrated to play essential roles in multiple liver diseases. Toll-interacting protein (Tollip) acts as an endogenous negative modulator of TLR signaling and is implicated in various cardio-metabolic diseases. However, the effect of Tollip in hepatocellular carcinoma (HCC) remains elusive. In the current study, enhanced Tollip expression was observed in HCC cells and tissues examined by RT-PCR, western blot, and immunohistochemistry staining. Moreover, the co-immunofluorescence staining demonstrated that increased Tollip expression was primarily located in hepatocytes. Functionally, Tollip overexpression significantly increased proliferation, migration, invasion, and epithelial-mesenchymal transition (EMT) of HCC cells, which ultimately accelerated tumorigenesis. Mechanistically, Tollip overexpression dramatically promoted the activation of PI3K/AKT signaling pathway in HCC cells which was attenuated by Tollip silencing. Importantly, the inhibition of PI3K/AKT axis can abolish the promoted effects of Tollip on proliferation and EMT of HCC cells. Our current study demonstrated that Tollip played an important role in the regulation of HCC development by engaging PI3K/AKT signaling pathway. These evidences suggested that the blockade of Tollip-PI3K/AKT axis was an ideal therapeutic treatment for management of HCC.
Keywords: PI3K/AKT; Tollip; epithelial-mesenchymal transition; hepatocellular carcinoma; prognosis.
© 2022 Lu Huang et al., published by De Gruyter.
Conflict of interest statement
Conflict of interest: None declared.
Figures





References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. - PubMed
-
- Cai Z, Liu Q. Understanding the Global Cancer Statistics 2018: implications for cancer control. Sci China Life Sci. 2021 Jun;64(6):1017–20. - PubMed
-
- Wallace MC, Preen D, Jeffrey GP, Adams LA. The evolving epidemiology of hepatocellular carcinoma: a global perspective. Expert Rev Gastroenterol Hepatol. 2015;9(6):765–79. - PubMed
LinkOut - more resources
Full Text Sources
