A Redox-Active Organic Cation for Safer Metallic Lithium-Based Batteries
- PMID: 35434389
- PMCID: PMC9012240
- DOI: 10.1016/j.ensm.2020.07.038
A Redox-Active Organic Cation for Safer Metallic Lithium-Based Batteries
Abstract
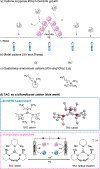
Safety concerns have severely impeded the practical application of high-energy-density lithium-based batteries. Dendrite growth and overcharging can lead to particularly catastrophic thermal failure. Here we report an organic cation, trisaminocyclopropenium (TAC), as a bi-functional electrolyte additive to suppress dendrite growth and offer reversible overcharge protection for metallic lithium-based batteries. During the Li plating process, TAC cations with aliphatic chains can form a positively charged electrostatic shield around Li protrusions, repelling the approaching Li+ and thereby attaining a more uniform plating. A two times longer cycle life of 300 h at 1 mA cm-2 is achieved in a Li|Li symmetric cell in comparison with the control. During the overcharging process, the redox-active TAC can repeatedly shuttle between two electrodes, maintaining the cell voltage within a safe value. A solid protection of 117 cycles (~1640 h) at 0.2 C with a 100% overcharge is achieved in a LiFePO4/Li4Ti5O12 cell. This study sheds fresh light on the ability of organic cations to build safer batteries.
Keywords: dendrite suppression; electrolyte additive; metallic lithium-based batteries; overcharge protection; trisaminocyclopropenium cation.
Figures
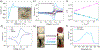


References
-
- Bruce PG, Freunberger SA, Hardwick LJ, Tarascon J-M, Nat. Mater 11 (2012) 19–29. - PubMed
-
- Wang Q, Ping P, Zhao X, Chu G, Sun J, Chen C, J. Power Sources 208 (2012) 210–224
-
- Feng X, Ouyang M, Liu X, Lu L, Xia Y, He X, Energy Storage Mater 10 (2018) 246–267
-
- Kushima A, So KP, Su C, Bai P, Kuriyama N, Maebashi T, Fujiwara Y, Bazant MZ, Li J, Nano Energy 32 (2017) 271–279
Grants and funding
LinkOut - more resources
Full Text Sources
