Comprehensive Commissioning and Clinical Implementation of GammaTiles STaRT for Intracranial Brain Cancer
- PMID: 35434425
- PMCID: PMC9010698
- DOI: 10.1016/j.adro.2022.100910
Comprehensive Commissioning and Clinical Implementation of GammaTiles STaRT for Intracranial Brain Cancer
Abstract
Purpose: To validate the dose calculation accuracy and dose distribution of GammaTiles for brain tumors, and to suggest a surgically targeted radiation therapy (STaRT) workflow for planning, delivery, radiation safety documentation, and posttreatment validation.
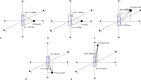
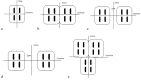
Methods and materials: Novel surgically targeted radiation therapy, GammaTiles, uses Cs-131 radiation isotopes embedded in collagen-based tiles that can be resorbed after surgery. GammaTile target delineation and dose calculation were performed on MIM Symphony software. Point-based and complex seed distribution calculations in MIM Symphony were verified with hand calculations and BrachyVision calculations. Vendor-provided 2-dimensional dose distribution calculation accuracy was validated using gafchromic EBT3 film measurements at various depths. A workflow was established for safe and effective GammaTile implants.
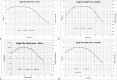
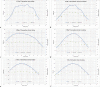
Results: Good agreement was observed between different calculations. Calculation accuracy of less than 0.5% was achieved for all points except one, which had rounding issues for very low doses and resulted in just below 5% difference. Differences in anisotropy and geometry positioning were noticed in the delineation of Cs-131 IsoRay seeds in the compared systems, resulting in minor discrepancies in the calculated dosimetry distributions. Film measurements showed profiles with relatively good agreement of 0% to 5% in nongradient regions with higher differences between 5% to 10% in the sharp dose fall-off regions.
Conclusions: A comprehensive evaluation of GammaTile geometry, dose distribution, and clinical workflow was conducted. Safe intro-operative implantation of GammaTiles requires extensive preplanning and interdisciplinary collaboration. A STaRT workflow was outlined to provide a guideline for an accurate treatment planning and safe implant process at other institutions.
© 2022 The Author(s).
Figures

References
-
- DeAngelis LM. Brain tumors. N Engl J Med. 2001;344:114–123. - PubMed
-
- Ohgaki H, Kleihues P. The definition of primary and secondary glioblastoma. Clin Cancer Res. 2013;19:764–772. - PubMed
-
- Ali-Reza F, Ulrich R. Meningioma. Curr Neurol Neurosci Rep. 2013;13:337. - PubMed
-
- Wen PY, Black PM, Loeffler JS. In: Cancer: Principles and Practice of Oncology. DeVita V, Hellman S, Rosenberg SA, editors. Lippincott, Williams, & Wilkins; Philadelphia, PA: 2001. Metastatic brain cancer; pp. 2655–2670.
LinkOut - more resources
Full Text Sources

