Comprehensive Preclinical Assessment of Sensory, Functional, Motivational-Affective, and Neurochemical Outcomes in Neuropathic Pain: The Case of the Sigma-1 Receptor
- PMID: 35434530
- PMCID: PMC9003638
- DOI: 10.1021/acsptsci.2c00005
Comprehensive Preclinical Assessment of Sensory, Functional, Motivational-Affective, and Neurochemical Outcomes in Neuropathic Pain: The Case of the Sigma-1 Receptor
Abstract
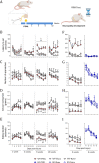
Chronic pain remains a major health problem and is currently facing slow drug innovation. New drug treatments should address not only the sensory-discriminative but also functional and motivational-affective components of chronic pain. In a mouse model of neuropathic pain induced by partial sciatic nerve ligation (PSNL), we analyzed sensory and functional-like outcomes by hindpaw mechanical stimulation and automated gait analysis (CatWalk). We characterized over time a reward-seeking task based on diminished motivation for natural reinforcers (anhedonic-like behavior). To differentiate the appetitive ("wanting") and consummatory ("liking") aspects of motivational behavior, we quantified the latency and number of approaches to eat white chocolate, as well as the eating duration and amount consumed. We explored a putative chronic pain-induced dysregulation of monoamine function by measuring monoamine levels in the nucleus accumbens (NAc), a well-known brain reward area. Finally, we investigated the role of sigma-1 receptor (σ1R) modulation, a nonopioid target, in these multiple dimensions by genetic deletion and pharmacological dose-response studies. After 6 weeks, PSNL increased the approach latency and reduced the consumption of white chocolate in 20-25% of the mice, while around 50-60% had one or the other parameter affected independently. After 10 weeks, sham-operated mice also displayed anhedonic-like behavior. PSNL was associated with reduced extracellular baseline dopamine and increased norepinephrine in the NAc and with a suppression of increased dopamine and serotonin efflux in response to the rewarding stimulus. Genetic and pharmacological blockade of σ1R relieved these multiple alterations in nerve-injured mice. We comprehensively describe sensory, functional, and depression-like impairment of key components of motivated behavior associated with nerve injury. We provide a neurochemical substrate for the depressed mesocorticolimbic reward processing in chronic pain, with a potentially increased translational value. Our results also highlight σ1R for the therapeutic intervention of neuropathic pain.
© 2022 American Chemical Society.
Conflict of interest statement
The authors declare the following competing financial interest(s): All authors are currently full-time employees of Welab Barcelona and formerly full-time employees of ESTEVE Pharmaceuticals, when the experimental work was performed.
Figures



References
-
- Treede R.-D.; Rief W.; Barke A.; Aziz Q.; Bennett M. I.; Benoliel R.; Cohen M.; Evers S.; Finnerup N. B.; First M. B.; Giamberardino M. A.; Kaasa S.; Korwisi B.; Kosek E.; Lavand’homme P.; Nicholas M.; Perrot S.; Scholz J.; Schug S.; Smith B. H.; Svensson P.; Vlaeyen J. W. S.; Wang S.-J. Chronic Pain as a Symptom or a Disease: The IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain 2019, 160, 19–27. 10.1097/j.pain.0000000000001384. - DOI - PubMed
-
- Busse J. W.; Wang L.; Kamaleldin M.; Craigie S.; Riva J. J.; Montoya L.; Mulla S. M.; Lopes L. C.; Vogel N.; Chen E.; Kirmayr K.; De Oliveira K.; Olivieri L.; Kaushal A.; Chaparro L. E.; Oyberman I.; Agarwal A.; Couban R.; Tsoi L.; Lam T.; Vandvik P. O.; Hsu S.; Bala M. M.; Schandelmaier S.; Scheidecker A.; Ebrahim S.; Ashoorion V.; Rehman Y.; Hong P. J.; Ross S.; Johnston B. C.; Kunz R.; Sun X.; Buckley N.; Sessler D. I.; Guyatt G. H. Opioids for Chronic Noncancer Pain: A Systematic Review and Meta-Analysis. JAMA 2018, 320, 2448.10.1001/jama.2018.18472. - DOI - PMC - PubMed
-
- Kleykamp B. A.; Ferguson M. C.; McNicol E.; Bixho I.; Arnold L. M.; Edwards R. R.; Fillingim R.; Grol-Prokopczyk H.; Turk D. C.; Dworkin R. H. The Prevalence of Psychiatric and Chronic Pain Comorbidities in Fibromyalgia: An ACTTION Systematic Review. Semin. Arthritis Rheum. 2021, 51, 166–174. 10.1016/j.semarthrit.2020.10.006. - DOI - PubMed
LinkOut - more resources
Full Text Sources
