Peptide-based delivery of therapeutics in cancer treatment
- PMID: 35434595
- PMCID: PMC9010702
- DOI: 10.1016/j.mtbio.2022.100248
Peptide-based delivery of therapeutics in cancer treatment
Abstract
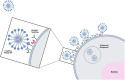
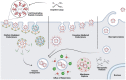
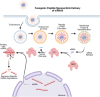
Current delivery strategies for cancer therapeutics commonly cause significant systemic side effects due to required high doses of therapeutic, inefficient cellular uptake of drug, and poor cell selectivity. Peptide-based delivery systems have shown the ability to alleviate these issues and can significantly enhance therapeutic loading, delivery, and cancer targetability. Peptide systems can be tailor-made for specific cancer applications. This review describes three peptide classes, targeting, cell penetrating, and fusogenic peptides, as stand-alone nanoparticle systems, conjugations to nanoparticle systems, or as the therapeutic modality. Peptide nanoparticle design, characteristics, and applications are discussed as well as peptide applications in the clinical space.
Keywords: Cancer; Drug therapy; Gene therapy; Nanoparticle conjugation; Peptide delivery.
© 2022 Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

