Dosing interval strategies for two-dose COVID-19 vaccination in 13 middle-income countries of Europe: Health impact modelling and benefit-risk analysis
- PMID: 35434685
- PMCID: PMC8996067
- DOI: 10.1016/j.lanepe.2022.100381
Dosing interval strategies for two-dose COVID-19 vaccination in 13 middle-income countries of Europe: Health impact modelling and benefit-risk analysis
Abstract
Background: In settings where the COVID-19 vaccine supply is constrained, extending the intervals between the first and second doses of the COVID-19 vaccine may allow more people receive their first doses earlier. Our aim is to estimate the health impact of COVID-19 vaccination alongside benefit-risk assessment of different dosing intervals in 13 middle-income countries (MICs) of Europe.
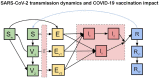
Methods: We fitted a dynamic transmission model to country-level daily reported COVID-19 mortality in 13 MICs in Europe (Albania, Armenia, Azerbaijan, Belarus, Bosnia and Herzegovina, Bulgaria, Georgia, Republic of Moldova, Russian Federation, Serbia, North Macedonia, Turkey, and Ukraine). A vaccine product with characteristics similar to those of the Oxford/AstraZeneca COVID-19 (AZD1222) vaccine was used in the base case scenario and was complemented by sensitivity analyses around efficacies similar to other COVID-19 vaccines. Both fixed dosing intervals at 4, 8, 12, 16, and 20 weeks and dose-specific intervals that prioritise specific doses for certain age groups were tested. Optimal intervals minimise COVID-19 mortality between March 2021 and December 2022. We incorporated the emergence of variants of concern (VOCs) into the model and conducted a benefit-risk assessment to quantify the tradeoff between health benefits versus adverse events following immunisation.
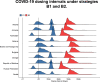
Findings: In all countries modelled, optimal strategies are those that prioritise the first doses among older adults (60+ years) or adults (20+ years), which lead to dosing intervals longer than six months. In comparison, a four-week fixed dosing interval may incur 10.1% [range: 4.3% - 19.0%; n = 13 (countries)] more deaths. The rapid waning of the immunity induced by the first dose (i.e. with means ranging 60-120 days as opposed to 360 days in the base case) resulted in shorter optimal dosing intervals of 8-20 weeks. Benefit-risk ratios were the highest for fixed dosing intervals of 8-12 weeks.
Interpretation: We infer that longer dosing intervals of over six months could reduce COVID-19 mortality in MICs of Europe. Certain parameters, such as rapid waning of first-dose induced immunity and increased immune escape through the emergence of VOCs, could significantly shorten the optimal dosing intervals.
Funding: World Health Organization.
Keywords: AEFI, Adverse events following immunisation; COVID-19; MIC, Middle income country; Mathematical modelling; Public health intervention; Quantitative methods; SARS-CoV-2; VE, Vaccine Efficacy; VOC, Variant of Concern; Vaccine policy.
© 2022 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
We declare no competing interests.
Figures



References
-
- CEPI, Gavi, UNICEF, WHO. COVAX global supply forecast [Internet]. 2021 [cited 2021 Sep 16]. Available from: https://www.gavi.org/news/document-library/covax-global-supply-forecast
-
- Iacobucci G., Mahase E. Covid-19 vaccination: what's the evidence for extending the dosing interval? BMJ. 2021;372:n18. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

