In-hospital mortality and severe outcomes after hospital discharge due to COVID-19: A prospective multicenter study from Brazil
- PMID: 35434696
- PMCID: PMC9001143
- DOI: 10.1016/j.lana.2022.100244
In-hospital mortality and severe outcomes after hospital discharge due to COVID-19: A prospective multicenter study from Brazil
Erratum in
-
Correction to "In-hospital mortality and severe outcomes after hospital discharge due to COVID-19: A prospective multicenter study from Brazil" [Lancet Reg Health Am. 2022 Jul; 11:100244] DOI: 10.1016/j.lana.2022.100244.Lancet Reg Health Am. 2022 Jul;11:100300. doi: 10.1016/j.lana.2022.100300. Epub 2022 Jun 17. Lancet Reg Health Am. 2022. PMID: 35756091 Free PMC article.
Abstract
Background: We evaluated in-hospital mortality and outcomes incidence after hospital discharge due to COVID-19 in a Brazilian multicenter cohort.
Methods: This prospective multicenter study (RECOVER-SUS, NCT04807699) included COVID-19 patients hospitalized in public tertiary hospitals in Brazil from June 2020 to March 2021. Clinical assessment and blood samples were performed at hospital admission, with post-hospital discharge remote visits. Hospitalized participants were followed-up until March 31, 2021. The outcomes were in-hospital mortality and incidence of rehospitalization or death after hospital discharge. Kaplan-Meier curves and Cox proportional-hazard models were performed.
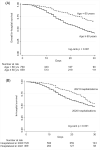
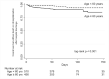
Findings: 1589 participants [54.5% male, age=62 (IQR 50-70) years; BMI=28.4 (IQR,24.9-32.9) Kg/m² and 51.9% with diabetes] were included. A total of 429 individuals [27.0% (95%CI,24.8-29.2)] died during hospitalization (median time 14 (IQR,9-24) days). Older age [vs<40 years; age=60-69 years-aHR=1.89 (95%CI,1.08-3.32); age=70-79 years-aHR=2.52 (95%CI,1.42-4.45); age≥80-aHR=2.90 (95%CI 1.54-5.47)]; noninvasive or mechanical ventilation at admission [vs facial-mask or none; aHR=1.69 (95%CI 1.30-2.19)]; SAPS-III score≥57 [vs<57; aHR=1.47 (95%CI 1.13-1.92)] and SOFA score≥10 [vs <10; aHR=1.51 (95%CI 1.08-2.10)] were independently associated with in-hospital mortality. A total of 65 individuals [6.7% (95%CI 5.3-8.4)] had a rehospitalization or death [rate=323 (95%CI 250-417) per 1000 person-years] in a median time of 52 (range 1-280) days post-hospital discharge. Age ≥ 60 years [vs <60, aHR=2.13 (95%CI 1.15-3.94)] and SAPS-III ≥57 at admission [vs <57, aHR=2.37 (95%CI 1.22-4.59)] were independently associated with rehospitalization or death after hospital discharge.
Interpretation: High in-hospital mortality rates due to COVID-19 were observed and elderly people remained at high risk of rehospitalization and death after hospital discharge.
Funding: Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Programa INOVA-FIOCRUZ.
Keywords: ALT, alanine aminotransferase; AST, aspartate aminotransferase BMI, body mass index; CI, confidence interval; COVID-19; COVID-19, Coronavirus disease 2019; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; ICU, intensive care unit; INR, international normalized ratio; IQR, interquartile range; In-hospital mortality; NIV, non-invasive ventilation; PD, person-days; PY, person-years; Post-COVID-19; REDCap, research electronic data capture; SAPS-III, simplified acute physiology score III; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SOFA, sequential organ failure assessment; VIF, variance inflation factor; VOC, variant of concern; WHO, World Health Organization; aHR, adjusted-hazard ratio.
© 2022 The Author(s).
Conflict of interest statement
Estevão Portela Nunes has received payment for lectures by Gilead; Alexandre Vargas Schwarzbold has received grants from AZ, MSD and Clover Biopharm; Fernanda Carvalho de Queiroz Mello has been acting as the President of the Society of Pneumonology and Tisiology fo the State of Rio de Janeiro (no payment) and Beatriz Grinsztejn has been participating in Advisory Board of Merck; GSK/ViiV and Janssen; The other authors declare no conflicts of interest.
Figures
References
-
- Teixeira M.G., Costa M., Paixao E.S.D., Carmo E.H., Barreto F.R., Penna G.O. The achievements of the SUS in tackling the communicable diseases. Cienc Saude Colet. 2018;23(6):1819–1828. - PubMed
-
- Coronavirus Pandemic (COVID-19) – the data URL: https://ourworldindata.org/coronavirus-data. Acessed 8 March 2022
-
- Kim L., Garg S., O'Halloran A., et al. Risk factors for intensive care unit admission and in-hospital mortality among hospitalized adults identified through the US coronavirus disease 2019 (COVID-19)-associated hospitalization surveillance network (COVID-NET) Clin Infect Dis. 2021;72(9):e206–e214. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

