Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)-Specific T Cells and Antibodies in Coronavirus Disease 2019 (COVID-19) Protection: A Prospective Study
- PMID: 35435222
- PMCID: PMC9047235
- DOI: 10.1093/cid/ciac278
Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2)-Specific T Cells and Antibodies in Coronavirus Disease 2019 (COVID-19) Protection: A Prospective Study
Abstract
Background: During the ongoing coronavirus disease 2019 (COVID-19) pandemic, many individuals were infected with and have cleared the virus, developing virus-specific antibodies and effector/memory T cells. An important unanswered question is what levels of T-cell and antibody responses are sufficient to protect from the infection.
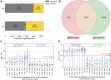
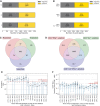
Methods: In 5340 Moscow residents, we evaluated anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) immunoglobulin M (IgM)/immunoglobulin G (IgG) titers and frequencies of the T cells specific to the membrane, nucleocapsid, and spike proteins of SARS-CoV-2, using interferon gamma (IFN-γ) enzyme-linked immunosorbent spot (ELISpot) assay. Additionally, we evaluated the fractions of virus-specific CD4+ and CD8+ T cells using intracellular staining of IFN-γ and interleukin 2 followed by flow cytometry. We analyzed the COVID-19 rates as a function of the assessed antibody and T-cell responses, using the Kaplan-Meier estimator method, for up to 300 days postinclusion.
Results: We showed that T-cell and antibody responses are closely interconnected and are commonly induced concurrently. Magnitudes of both responses inversely correlated with infection probability. Individuals positive for both responses demonstrated the highest levels of protectivity against the SARS-CoV-2 infection. A comparable level of protection was found in individuals with antibody response only, whereas the T-cell response by itself granted only intermediate protection.
Conclusions: We found that the contribution of the virus-specific antibodies to protection against SARS-CoV-2 infection is more pronounced than that of the T cells. The data on the virus-specific IgG titers may be instructive for making decisions in personalized healthcare and public anti-COVID-19 policies. Clinical Trials Registration. NCT04898140.
Keywords: COVID-19; SARS-CoV-2; T cells; immune response; protective effect.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Infectious Diseases Society of America.
Conflict of interest statement
Conflicts of interest. The authors declare no competing interests. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Figures


References
-
- Le Bert N, Tan AT, Kunasegaran K, et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature 2020; 584:457–62. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

