Immunotherapy of Neuroblastoma: Facts and Hopes
- PMID: 35435953
- PMCID: PMC9344822
- DOI: 10.1158/1078-0432.CCR-21-1356
Immunotherapy of Neuroblastoma: Facts and Hopes
Abstract
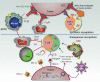
While the adoption of multimodal therapy including surgery, radiation, and aggressive combination chemotherapy has improved outcomes for many children with high-risk neuroblastoma, we appear to have reached a plateau in what can be achieved with cytotoxic therapies alone. Most children with cancer, including high-risk neuroblastoma, do not benefit from treatment with immune checkpoint inhibitors (ICI) that have revolutionized the treatment of many highly immunogenic adult solid tumors. This likely reflects the low tumor mutation burden as well as the downregulated MHC-I that characterizes most high-risk neuroblastomas. For these reasons, neuroblastoma represents an immunotherapeutic challenge that may be a model for the creation of effective immunotherapy for other "cold" tumors in children and adults that do not respond to ICI. The identification of strong expression of the disialoganglioside GD2 on the surface of nearly all neuroblastoma cells provided a target for immune recognition by anti-GD2 mAbs that recruit Fc receptor-expressing innate immune cells that mediate cytotoxicity or phagocytosis. Adoption of anti-GD2 antibodies into both upfront and relapse treatment protocols has dramatically increased survival rates and altered the landscape for children with high-risk neuroblastoma. This review describes how these approaches have been expanded to additional combinations and forms of immunotherapy that have already demonstrated clear clinical benefit. We also describe the efforts to identify additional immune targets for neuroblastoma. Finally, we summarize newer approaches being pursued that may well help both innate and adaptive immune cells, endogenous or genetically engineered, to more effectively destroy neuroblastoma cells, to better induce complete remission and prevent recurrence.
©2022 The Authors; Published by the American Association for Cancer Research.
Figures

References
-
- Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. . Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016;375:1823–33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

