Humoral Responses Against SARS-CoV-2 and Variants of Concern After mRNA Vaccines in Patients With Non-Hodgkin Lymphoma and Chronic Lymphocytic Leukemia
- PMID: 35436146
- PMCID: PMC9470134
- DOI: 10.1200/JCO.22.00088
Humoral Responses Against SARS-CoV-2 and Variants of Concern After mRNA Vaccines in Patients With Non-Hodgkin Lymphoma and Chronic Lymphocytic Leukemia
Abstract
Purpose: Patients with non-Hodgkin lymphoma including chronic lymphocytic leukemia (NHL/CLL) are at higher risk of severe SARS-CoV-2 infection. We investigated vaccine-induced antibody responses in patients with NHL/CLL against the original SARS-CoV-2 strain and variants of concern including B.1.167.2 (Delta) and B.1.1.529 (Omicron).
Materials and methods: Blood from 121 patients with NHL/CLL receiving two doses of vaccine were collected longitudinally. Antibody binding against the full-length spike protein, the receptor-binding, and N-terminal domains of the original strain and of variants was measured using a multiplex assay. Live-virus neutralization against Delta, Omicron, and the early WA1/2020 strains was measured using a focus reduction neutralization test. B cells were measured by flow cytometry. Correlation between vaccine response and clinical factors was determined.
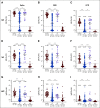
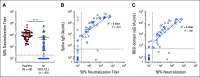
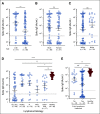
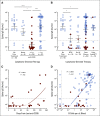
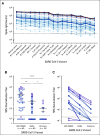
Results: Mean anti-SARS-CoV-2 spike immunoglobulin G-binding titers were 85-fold lower in patients with NHL/CLL compared with healthy controls, with seroconversion occurring in only 67% of patients. Neutralization titers were also lower and correlated with binding titers (P < .0001). Treatment with anti-CD20-directed therapies within 1 year resulted in 136-fold lower binding titers. Peripheral blood B-cell count also correlated with vaccine response. At 3 months from last anti-CD20-directed therapy, B-cell count ≥ 4.31/μL blood around the time of vaccination predicted response (OR 7.46, P = .04). Antibody responses also correlated with age. Importantly, neutralization titers against Delta and Omicron were reduced six- and 42-fold, respectively, with 67% of patients seropositive for WA1/2020 exhibiting seronegativity for Omicron.
Conclusion: Antibody binding and live-virus neutralization against SARS-CoV-2 and its variants of concern including Delta and Omicron were substantially lower in patients with NHL/CLL compared with healthy vaccinees. Anti-CD20-directed therapy < 1 year before vaccination and number of circulating B cells strongly predict vaccine response.
Conflict of interest statement
Humoral Responses Against SARS-CoV-2 and Variants of Concern After mRNA Vaccines in Patients With Non-Hodgkin Lymphoma and Chronic Lymphocytic Leukemia
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures

References
-
- Cattaneo C, Daffini R, Pagani C, et al. Clinical characteristics and risk factors for mortality in hematologic patients affected by COVID-19. Cancer. 2020;126:5069–5076. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

