Live imaging of the Cryptosporidium parvum life cycle reveals direct development of male and female gametes from type I meronts
- PMID: 35436284
- PMCID: PMC9015140
- DOI: 10.1371/journal.pbio.3001604
Live imaging of the Cryptosporidium parvum life cycle reveals direct development of male and female gametes from type I meronts
Abstract
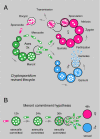
Cryptosporidium is a leading infectious cause of diarrhea around the world associated with waterborne outbreaks, community spread, or zoonotic transmission. The parasite has significant impact on early childhood mortality, and infection is both a consequence and cause of malnutrition and stunting. There is currently no vaccine, and treatment options are very limited. Cryptosporidium is a member of the Apicomplexa, and, as typical for this, protist phylum relies on asexual and sexual reproduction. In contrast to other Apicomplexa, including the malaria parasite Plasmodium, the entire Cryptosporidium life cycle unfolds in a single host in less than 3 days. Here, we establish a model to image life cycle progression in living cells and observe, track, and compare nuclear division of asexual and sexual stage parasites. We establish the length and sequence of the cell cycles of all stages and map the developmental fate of parasites across multiple rounds of invasion and egress. We propose that the parasite executes an intrinsic program of 3 generations of asexual replication, followed by a single generation of sexual stages that is independent of environmental stimuli. We find no evidence for a morphologically distinct intermediate stage (the tetraploid type II meront) but demonstrate direct development of gametes from 8N type I meronts. The progeny of each meront is collectively committed to either asexual or sexual fate, but, importantly, meronts committed to sexual fate give rise to both males and females. We define a Cryptosporidium life cycle matching Tyzzer's original description and inconsistent with the coccidian life cycle now shown in many textbooks.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Comment in
-
3, 2, 1, go! Cryptosporidium counts down to sex.PLoS Biol. 2022 May 12;20(5):e3001638. doi: 10.1371/journal.pbio.3001638. eCollection 2022 May. PLoS Biol. 2022. PMID: 35552541 Free PMC article.
References
-
- Collaborators GBDDD. Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017;17(9):909–48. Epub 2017/06/06. doi: 10.1016/S1473-3099(17)30276-1 ; PubMed Central PMCID: PMC5589208. - DOI - PMC - PubMed
-
- Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, et al.. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382(9888):209–22. Epub 2013/05/18. doi: 10.1016/S0140-6736(13)60844-2 . - DOI - PubMed
-
- Checkley W, White AC Jr, Jaganath D, Arrowood MJ, Chalmers RM, Chen XM, et al.. A review of the global burden, novel diagnostics, therapeutics, and vaccine targets for cryptosporidium. Lancet Infect Dis. 2015;15(1):85–94. doi: 10.1016/S1473-3099(14)70772-8 ; PubMed Central PMCID: PMC4401121. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

