Association between paracetamol use during pregnancy and perinatal outcomes: Prospective NISAMI cohort
- PMID: 35436308
- PMCID: PMC9015137
- DOI: 10.1371/journal.pone.0267270
Association between paracetamol use during pregnancy and perinatal outcomes: Prospective NISAMI cohort
Abstract
Background: Paracetamol is widely used to manage fever and pain during pregnancy worldwide. However, paracetamol may affect the pregnant woman and fetus, once this drug crosses the placental barrier after therapeutic doses and may impair fetal liver function, affecting fetus growth and development. Thus, this study aimed to investigate the association between paracetamol use during pregnancy and perinatal outcomes as preterm birth, low birth weight, and small for gestational age.
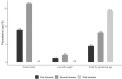
Methods and findings: Data from 760 pregnant women within the NISAMI Cohort between June 2012 and February 2014 were analyzed. Logistic regression was used to estimate the association among paracetamol use during pregnancy and preterm birth, low birth weight, and small for gestational age. Multivariate analyses were adjusted for socioeconomic, maternal, pregnancy, and newborn covariates. Around 14% of women were exposed to paracetamol during pregnancy. A decrease in paracetamol use throughout pregnancy was observed. Lower risk of low birth weight in infants born to women exposed to the drug (OR 0.21; IC 95% 0.01-0.99) was found. Paracetamol use during pregnancy was not statistically associated with preterm birth or small for gestational age.
Conclusions: The findings of this study do not suggest an increased risk of perinatal outcomes. However, it should not be assumed that paracetamol is a risk-free medication and its use must be rational.
Conflict of interest statement
The authors declared that no competing interests exist.
Figures
Similar articles
-
Marijuana use in opioid exposed pregnancy increases risk of preterm birth.J Matern Fetal Neonatal Med. 2022 Dec;35(25):8456-8461. doi: 10.1080/14767058.2021.1980532. Epub 2021 Sep 28. J Matern Fetal Neonatal Med. 2022. PMID: 34582287
-
Pregnancy outcomes in nulliparous women with positive first-trimester preterm preeclampsia screening test: the Great Obstetrical Syndromes cohort study.Am J Obstet Gynecol. 2021 Feb;224(2):204.e1-204.e7. doi: 10.1016/j.ajog.2020.08.008. Epub 2020 Aug 7. Am J Obstet Gynecol. 2021. PMID: 32777265
-
Association between maternal multimorbidity and preterm birth, low birth weight and small for gestational age: a prospective birth cohort study from the Japan Environment and Children's Study.BMJ Open. 2023 Mar 15;13(3):e069281. doi: 10.1136/bmjopen-2022-069281. BMJ Open. 2023. PMID: 36921942 Free PMC article.
-
Birth Outcomes of Neonates Exposed to Marijuana in Utero: A Systematic Review and Meta-analysis.JAMA Netw Open. 2022 Jan 4;5(1):e2145653. doi: 10.1001/jamanetworkopen.2021.45653. JAMA Netw Open. 2022. PMID: 35084479 Free PMC article.
-
Risk of stillbirth, preterm delivery, and fetal growth restriction following exposure in a previous birth: systematic review and meta-analysis.BJOG. 2018 Jan;125(2):183-192. doi: 10.1111/1471-0528.14906. Epub 2017 Oct 3. BJOG. 2018. PMID: 28856792
Cited by
-
Association of acetaminophen use with perinatal outcomes among pregnant women: a retrospective cohort study with propensity score matching.BMC Pregnancy Childbirth. 2024 Apr 11;24(1):268. doi: 10.1186/s12884-024-06480-5. BMC Pregnancy Childbirth. 2024. PMID: 38605288 Free PMC article.
-
Neonatal and maternal adverse outcomes and exposure to nonsteroidal anti-inflammatory drugs during early pregnancy in South Korea: A nationwide cohort study.PLoS Med. 2023 Feb 27;20(2):e1004183. doi: 10.1371/journal.pmed.1004183. eCollection 2023 Feb. PLoS Med. 2023. PMID: 36848338 Free PMC article.
-
Racial Disparities in Medication Use During Pregnancy: Results from the NISAMI Cohort.J Multidiscip Healthc. 2024 Jun 5;17:2755-2775. doi: 10.2147/JMDH.S455378. eCollection 2024. J Multidiscip Healthc. 2024. PMID: 38855020 Free PMC article.
-
Understanding Miscarriage Prevalence and Risk Factors: Insights from Women in Jordan.Medicina (Kaunas). 2024 Jun 26;60(7):1044. doi: 10.3390/medicina60071044. Medicina (Kaunas). 2024. PMID: 39064473 Free PMC article.
-
The relationship of prenatal acetaminophen exposure and attention-related behavior in early childhood.Neurotoxicol Teratol. 2024 Jan-Feb;101:107319. doi: 10.1016/j.ntt.2024.107319. Epub 2024 Jan 8. Neurotoxicol Teratol. 2024. PMID: 38199313 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources