Resistance to mogamulizumab is associated with loss of CCR4 in cutaneous T-cell lymphoma
- PMID: 35436328
- PMCID: PMC9247360
- DOI: 10.1182/blood.2021014468
Resistance to mogamulizumab is associated with loss of CCR4 in cutaneous T-cell lymphoma
Abstract
Mogamulizumab is a humanized anti-CC chemokine receptor 4 (CCR4) antibody approved for the treatment of mycosis fungoides and Sézary syndrome. Despite almost universal expression of CCR4 in these diseases, most patients eventually develop resistance to mogamulizumab. We tested whether resistance to mogamulizumab is associated with loss of CCR4 expression. We identified 17 patients with mycosis fungoides or Sézary syndrome who either were intrinsically resistant or acquired resistance to mogamulizumab. Low expression of CCR4 by immunohistochemistry or flow cytometry was found in 65% of patients. Novel emergent CCR4 mutations targeting the N-terminal and transmembrane domains were found in 3 patients after disease progression. Emerging CCR4 copy number loss was detected in 2 patients with CCR4 mutations. Acquisition of CCR4 genomic alterations corresponded with loss of CCR4 antigen expression. We also report on outcomes of 3 cutaneous T-cell lymphoma (CTCL) patients with gain-of-function CCR4 mutations treated with mogamulizumab. Our study indicates that resistance to mogamulizumab in CTCL frequently involves loss of CCR4 expression and emergence of CCR4 genomic alterations. This finding has implications for management and monitoring of CTCL patients on mogamulizumab and development of future CCR4-directed therapies.
© 2022 by The American Society of Hematology.
Figures
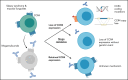
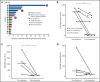
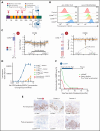
Comment in
-
Mechanisms of resistance to mogamulizumab.Blood. 2022 Jun 30;139(26):3674-3676. doi: 10.1182/blood.2022016594. Blood. 2022. PMID: 35771561 No abstract available.
References
-
- Ferenczi K, Fuhlbrigge RC, Pinkus J, Pinkus GS, Kupper TS. Increased CCR4 expression in cutaneous T cell lymphoma. J Invest Dermatol. 2002;119(6): 1405-1410. - PubMed
-
- Yoshie O, Matsushima K. CCR4 and its ligands: from bench to bedside. Int Immunol. 2015;27(1):11-20. - PubMed
-
- Kim YH, Bagot M, Pinter-Brown L, et al. . Mogamulizumab versus vorinostat in previously treated cutaneous T-cell lymphoma (MAVORIC): an international, open-label, randomised, controlled phase 3 trial. Lancet Oncol. 2018;19(9):1192-1204. - PubMed
-
- Kim YH, Khodadoust MS, de Masson A, et al. . Patient characteristics of long-term responders to mogamulizumab: results from the MAVORIC study. Eur J Cancer. 2021; 156(suppl 1):S48-S49. - PubMed
-
- Olsen EA, Whittaker S, Kim YH, et al. ; Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer . Clinical end points and response criteria in mycosis fungoides and Sézary syndrome: a consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J Clin Oncol. 2011;29(18):2598-2607. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

