Synthesis of Benzo[ b]thiophenes via Electrophilic Sulfur Mediated Cyclization of Alkynylthioanisoles
- PMID: 35436400
- PMCID: PMC9454903
- DOI: 10.1021/acs.joc.1c02606
Synthesis of Benzo[ b]thiophenes via Electrophilic Sulfur Mediated Cyclization of Alkynylthioanisoles
Abstract
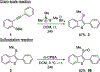
A stable dimethyl(thiodimethyl)sulfonium tetrafluoroborate salt was employed for the electrophilic cyclization reaction of o-alkynyl thioanisoles for the synthesis of 2,3-disubstituted benzo[b]thiophenes. The reaction described herein works well with various substituted alkynes in excellent yields, and a valuable thiomethyl group was introduced with ease. The reaction utilizes moderate reaction conditions and ambient temperature while tolerating various functionalities. To elucidate the mechanism, electrophilic addition reactions using the dimethyl(thiodimethyl)sulfonium tetrafluoroborate salt with diphenylacetylene was demonstrated.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

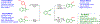




References
-
- Keri RS; Chand K; Budagumpi S; Balappa Somappa S; Patil SA; Nagaraja BM An Overview of Benzo[b]thiophene-Based Medicinal Chemistry. Eur. J. Med. Chem 2017, 138, 1002–1033. - PubMed
- Wishart DS; Feunang YD; Guo AC; Lo EJ; Marcu A; Grant JR; Sajed T; Johnson D; Li C; Sayeeda Z; Assempour N; Iynkkaran I; Liu Y; Maciejewski A; Gale N; Wilson A; Chin L; Cummings R; Le D; Pon A; Knox C; Wilson M DrugBank 5.0: A Major Update to the DrugBank Database for 2018. Nucleic Acids Res. 2018, 46 (D1), D1074–D1082. - PMC - PubMed
-
- Sweidan K; Engelmann J; Rayyan W; Sabbah D; Zarga M; Al-Qirim T; Al-Hiari Y; Sheikha G; Shattat G Synthesis and Preliminary Biological Evaluation of New Heterocyclic Carboxamide Models. Lett. Drug Des. Discovery 2015, 12 (5), 417–429.
- Martorana A; Gentile C; Perricone U; Piccionello AP; Bartolotta R; Terenzi A; Pace A; Mingoia F; Almerico AM; Lauria A Synthesis, Antiproliferative Activity, and in Silico Insights of New 3-Benzoylamino-Benzo[b]thiophene Derivatives. Eur. J. Med. Chem 2015, 90, 537–546. - PubMed
-
- Rosada B; Bekier A; Cytarska J; Płaziński W; Zavyalova O; Sikora A; Dzitko K; Łączkowski KZ Benzo[b]thiophene-Thiazoles as Potent Anti-Toxoplasma Gondii Agents: Design, Synthesis, Tyrosinase/tyrosine Hydroxylase Inhibitors, Molecular Docking Study, and Antioxidant Activity. Eur. J. Med. Chem 2019, 184, 111765. - PubMed
-
- Fakhr IMI; Radwan MAA; El-Batran S; Abd El-Salam OME; El-Shenawy SM Synthesis and Pharmacological Evaluation of 2-Substituted Benzo[b]thiophenes as Anti-Inflammatory and Analgesic Agents. Eur. J. Med. Chem 2009, 44 (4), 1718–1725. - PubMed
- Isloor AM; Kalluraya B; Sridhar Pai K Synthesis, Characterization and Biological Activities of Some New Benzo[b]thiophene Derivatives. Eur. J. Med. Chem 2010, 45 (2), 825–830. - PubMed
-
- Rao GK Synthesis, Antitubercular and Antibacterial Activities of Some Quinazolinone Analogs Substituted with Benzothiophene. Chem. Sci. J 2015, DOI: 10.4172/2150-3494.100092. - DOI
- Mahajan PS; Nikam MD; Nawale LU; Khedkar VM; Sarkar D; Gill CH Synthesis and Antitubercular Activity of New Benzo[b]thiophenes. ACS Med. Chem. Lett 2016, 7 (8), 751–756. - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

