Glycosylation of viral proteins: Implication in virus-host interaction and virulence
- PMID: 35436420
- PMCID: PMC9037552
- DOI: 10.1080/21505594.2022.2060464
Glycosylation of viral proteins: Implication in virus-host interaction and virulence
Abstract
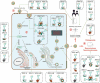
Glycans are among the most important cell molecular components. However, given their structural diversity, their functions have not been fully explored. Glycosylation is a vital post-translational modification for various proteins. Many bacteria and viruses rely on N-linked and O-linked glycosylation to perform critical biological functions. The diverse functions of glycosylation on viral proteins during viral infections, including Dengue, Zika, influenza, and human immunodeficiency viruses as well as coronaviruses have been reported. N-linked glycosylation is the most common form of protein modification, and it modulates folding, transportation and receptor binding. Compared to N-linked glycosylation, the functions of O-linked viral protein glycosylation have not been comprehensively evaluated. In this review, we summarize findings on viral protein glycosylation, with particular attention to studies on N-linked glycosylation in viral life cycles. This review informs the development of virus-specific vaccines or inhibitors.
Keywords: Glycosylation; viral nonstructural protein; viral pathogenesis; viral structural protein; virus life cycle.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical