SARS-CoV-2 Omicron triggers cross-reactive neutralization and Fc effector functions in previously vaccinated, but not unvaccinated, individuals
- PMID: 35436444
- PMCID: PMC8947963
- DOI: 10.1016/j.chom.2022.03.029
SARS-CoV-2 Omicron triggers cross-reactive neutralization and Fc effector functions in previously vaccinated, but not unvaccinated, individuals
Abstract
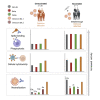
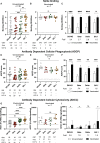
The SARS-CoV-2 Omicron variant escapes neutralizing antibodies elicited by vaccines or infection. However, whether Omicron triggers cross-reactive humoral responses to other variants of concern (VOCs) remains unknown. We used plasma from 20 unvaccinated and 7 vaccinated individuals infected by Omicron BA.1 to test binding, Fc effector function, and neutralization against VOCs. In unvaccinated individuals, Fc effector function and binding antibodies targeted Omicron and other VOCs at comparable levels. However, Omicron BA.1-triggered neutralization was not extensively cross-reactive for VOCs (14- to 31-fold titer reduction), and we observed 4-fold decreased titers against Omicron BA.2. In contrast, vaccination followed by breakthrough Omicron infection associated with improved cross-neutralization of VOCs with titers exceeding 1:2,100. This has important implications for the vulnerability of unvaccinated Omicron-infected individuals to reinfection by circulating and emerging VOCs. Although Omicron-based immunogens might be adequate boosters, they are unlikely to be superior to existing vaccines for priming in SARS-CoV-2-naive individuals.
Keywords: ADCC; ADCP; BA.1; BA.2; Omicron; SARS-CoV-2; breakthrough infection; neutralization; vaccines; variants of concern.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures

References
-
- Ackerman M.E., Moldt B., Wyatt R.T., Dugast A.-S., McAndrew E., Tsoukas S., Jost S., Berger C.T., Sciaranghella G., Liu Q., et al. A robust, high-throughput assay to determine the phagocytic activity of clinical antibody samples. J. Immunol. Methods. 2011;366:8–19. doi: 10.1016/j.jim.2010.12.016. - DOI - PMC - PubMed
-
- Bartsch Y., Tong X., Kang J., Avendaño M.J., Serrano E.F., García-Salum T., Pardo-Roa C., Riquelme A., Medina R.A., Alter G. Preserved Omicron spike specific antibody binding and Fc-recognition across COVID-19 vaccine platforms. medRxiv. 2021 doi: 10.1101/2021.12.24.21268378. Preprint at. - DOI
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

