SERPINA3C ameliorates adipose tissue inflammation through the Cathepsin G/Integrin/AKT pathway
- PMID: 35436587
- PMCID: PMC9062745
- DOI: 10.1016/j.molmet.2022.101500
SERPINA3C ameliorates adipose tissue inflammation through the Cathepsin G/Integrin/AKT pathway
Abstract
Objective: Due to the increasing prevalence of obesity and insulin resistance, there is an urgent need for better treatment of obesity and its related metabolic disorders. This study aimed to elucidate the role of SERPINA3C, an adipocyte secreted protein, in obesity and related metabolic disorders.
Methods: Male wild type (WT) and knockout (KO) mice were fed with high-fat diet (HFD) for 16 weeks, adiposity, insulin resistance, and inflammation were assessed. AAV-mediated overexpression of SERPINA3C was injected locally in inguinal white adipose tissue (iWAT) to examine the effect of SERPINA3C. In vitro analyses were conducted in 3T3-L1 adipocytes to explore the molecular pathways underlying the function of SERPINA3C.
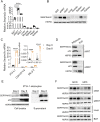
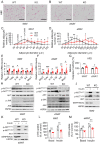
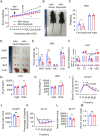
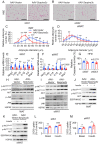
Results: Functional exploration of the SERPINA3C knockout mice revealed that SERPINA3C deficiency led to an impaired metabolic phenotype (more severe obesity, lower metabolic rates, worse glucose intolerance and insulin insensitivity), which was associated with anabatic inflammation and apoptosis of white adipose tissues. Consistent with these results, overexpression of SERPINA3C in inguinal adipose tissue protected mice against diet-induced obesity and metabolic disorders with less inflammation and apoptosis in adipose tissue. Mechanistically, SERPINA3C inhibited Cathepsin G activity, acting as a serine protease inhibitor, which blocked Cathepsin G-mediated turnover of α5/β1 Integrin protein. Then, the preserved integrity (increase) of α5/β1 Integrin signaling activated AKT to decrease JNK phosphorylation, thereby inhibiting inflammation and promoting insulin sensitivity in adipocytes.
Conclusions/interpretation: These findings demonstrate a previously unknown SERPINA3C/Cathepsin G/Integrin/AKT pathway in regulating adipose tissue inflammation, and suggest the therapeutic potential of targeting SERPINA3C/Cathepsin G axis in adipose tissue for the treatment of obesity and metabolic diseases.
Keywords: Adipokine; Inflammation; Insulin resistance; Integrin α5/β1; White adipose tissue.
Copyright © 2022 The Authors. Published by Elsevier GmbH.. All rights reserved.
Figures



References
-
- Ng A.C.T., Delgado V., Borlaug B.A., Bax J.J. Diabesity: the combined burden of obesity and diabetes on heart disease and the role of imaging. Nature Reviews Cardiology. 2021;18:291–304. - PubMed
-
- Peng W.Q., Xiao G., Li B.Y., Guo Y.Y., Guo L., Tang Q.Q. l-Theanine activates the browning of white adipose tissue through the AMPK/alpha-Ketoglutarate/Prdm16 Axis and ameliorates diet-induced obesity in mice. Diabetes. 2021;70:1458–1472. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

