Characterization of Hypothalamic MCH Neuron Development in a 3D Differentiation System of Mouse Embryonic Stem Cells
- PMID: 35437265
- PMCID: PMC9047030
- DOI: 10.1523/ENEURO.0442-21.2022
Characterization of Hypothalamic MCH Neuron Development in a 3D Differentiation System of Mouse Embryonic Stem Cells
Abstract
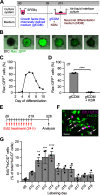
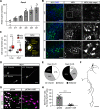
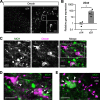
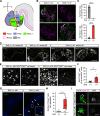
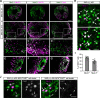
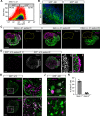
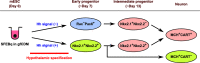
Hypothalamic melanin-concentrating hormone (MCH) neurons are important regulators of multiple physiological processes, such as sleep, feeding, and memory. Despite the increasing interest in their neuronal functions, the molecular mechanism underlying MCH neuron development remains poorly understood. We report that a three-dimensional culture of mouse embryonic stem cells (mESCs) can generate hypothalamic-like tissues containing MCH-positive neurons, which reproduce morphologic maturation, neuronal connectivity, and neuropeptide/neurotransmitter phenotype of native MCH neurons. Using this in vitro system, we demonstrate that Hedgehog (Hh) signaling serves to produce major neurochemical subtypes of MCH neurons characterized by the presence or absence of cocaine- and amphetamine-regulated transcript (CART). Without exogenous Hh signals, mESCs initially differentiated into dorsal hypothalamic/prethalamic progenitors and finally into MCH+CART+ neurons through a specific intermediate progenitor state. Conversely, activation of the Hh pathway specified ventral hypothalamic progenitors that generate both MCH+CART- and MCH+CART+ neurons. These results suggest that in vivo MCH neurons may originate from multiple cell lineages that arise through early dorsoventral patterning of the hypothalamus. Additionally, we found that Hh signaling supports the differentiation of mESCs into orexin/hypocretin neurons, a well-defined cell group intermingled with MCH neurons in the lateral hypothalamic area (LHA). The present study highlights and improves the utility of mESC culture in the analysis of the developmental programs of specific hypothalamic cell types.
Keywords: 3D culture; ES cells; MCH; hypothalamus; neuronal differentiation.
Copyright © 2022 Kodani et al.
Figures

Similar articles
-
Neurochemical Heterogeneity Among Lateral Hypothalamic Hypocretin/Orexin and Melanin-Concentrating Hormone Neurons Identified Through Single-Cell Gene Expression Analysis.eNeuro. 2017 Sep 22;4(5):ENEURO.0013-17.2017. doi: 10.1523/ENEURO.0013-17.2017. eCollection 2017 Sep-Oct. eNeuro. 2017. PMID: 28966976 Free PMC article.
-
Molecular codes and in vitro generation of hypocretin and melanin concentrating hormone neurons.Proc Natl Acad Sci U S A. 2019 Aug 20;116(34):17061-17070. doi: 10.1073/pnas.1902148116. Epub 2019 Aug 2. Proc Natl Acad Sci U S A. 2019. PMID: 31375626 Free PMC article.
-
Projection patterns of lateral hypothalamic, cocaine- and amphetamine-regulated transcript (CART) neurons to the dorsal raphe and/or the locus coeruleus in the rat.Brain Res. 2013 Feb 4;1494:56-69. doi: 10.1016/j.brainres.2012.11.042. Epub 2012 Dec 1. Brain Res. 2013. PMID: 23206801
-
The MCH neuron population as a model for the development and evolution of the lateral and dorsal hypothalamus.J Chem Neuroanat. 2016 Sep;75(Pt A):28-31. doi: 10.1016/j.jchemneu.2015.09.004. Epub 2015 Oct 13. J Chem Neuroanat. 2016. PMID: 26459022 Review.
-
Lateral hypothalamus as a sensor-regulator in respiratory and metabolic control.Physiol Behav. 2013 Sep 10;121:117-24. doi: 10.1016/j.physbeh.2013.03.023. Epub 2013 Apr 3. Physiol Behav. 2013. PMID: 23562864 Free PMC article. Review.
Cited by
-
Central Role of Hypothalamic Circuits for Acupuncture's Anti-Parkinsonian Effects.Adv Sci (Weinh). 2024 Nov;11(43):e2403245. doi: 10.1002/advs.202403245. Epub 2024 Aug 9. Adv Sci (Weinh). 2024. PMID: 39119926 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
