Partial Ablation of Astrocytes Exacerbates Cerebral Infiltration of Monocytes and Neuronal Loss After Brain Stab Injury in Mice
- PMID: 35437650
- PMCID: PMC11415208
- DOI: 10.1007/s10571-022-01224-5
Partial Ablation of Astrocytes Exacerbates Cerebral Infiltration of Monocytes and Neuronal Loss After Brain Stab Injury in Mice
Abstract
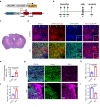
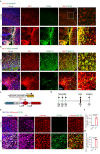
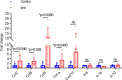
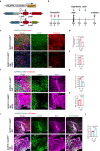
In traumatic brain injury (TBI), mechanical injury results in instantaneous tissue damages accompanied by subsequent pro-inflammatory cascades composed of microgliosis and astrogliosis. However, the interactive roles between microglia and astrocytes during the pathogenesis of TBI remain unclear and sometimes debatable. In this study, we used a forebrain stab injury mouse model to investigate the pathological role of reactive astrocytes in cellular and molecular changes of inflammatory response following TBI. In the ipsilateral hemisphere of stab-injured brain, monocyte infiltration and neuronal loss, as well as increased elevated astrogliosis, microglia activation and inflammatory cytokines were observed. To verify the role of reactive astrocytes in TBI, local and partial ablation of astrocytes was achieved by stereotactic injection of diphtheria toxin in the forebrain of Aldh1l1-CreERT2::Ai9::iDTR transgenic mice which expressed diphtheria toxin receptor (DTR) in astrocytes after tamoxifen induction. This strategy achieved about 20% of astrocytes reduction at the stab site as validated by immunofluorescence co-staining of GFAP with tdTomato-positive astrocytes. Interestingly, reduction of astrocytes showed increased microglia activation and monocyte infiltration, accompanied with increased severity in stab injury-induced neuronal loss when compared with DTR-/- mice, together with elevation of inflammatory chemokines such as CCL2, CCL5 and CXCL10 in astrogliosis-reduced mice. Collectively, our data verified the interactive role of astrocytes as an immune modulator in suppressing inflammatory responses in the injured brain. Schematic diagram shows monocyte infiltration and neuronal loss, as well as increased elevated astrogliosis, microglia activation and chemokines were observed in the injured site after stab injury. Local and partial ablation of astrocytes led to increased microglia activation and monocyte infiltration, accompanied with increased severity in neuronal loss together with elevation of inflammatory chemokines as compared with control mice subjected stab injury.
Keywords: Astrocytes; Microglia; Monocytes; Neuroinflammation; Stab injury; Traumatic brain injury.
© 2022. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
The authors have no conflict of interest.
Figures
Similar articles
-
Deletion of N-myc downstream-regulated gene 2 attenuates reactive astrogliosis and inflammatory response in a mouse model of cortical stab injury.J Neurochem. 2014 Aug;130(3):374-87. doi: 10.1111/jnc.12729. Epub 2014 Apr 25. J Neurochem. 2014. PMID: 24697507
-
Progranulin protects against exaggerated axonal injury and astrogliosis following traumatic brain injury.Glia. 2017 Feb;65(2):278-292. doi: 10.1002/glia.23091. Epub 2016 Oct 25. Glia. 2017. PMID: 27778404
-
Depletion of regulatory T cells increases T cell brain infiltration, reactive astrogliosis, and interferon-γ gene expression in acute experimental traumatic brain injury.J Neuroinflammation. 2019 Aug 5;16(1):163. doi: 10.1186/s12974-019-1550-0. J Neuroinflammation. 2019. PMID: 31383034 Free PMC article.
-
Cellular infiltration in traumatic brain injury.J Neuroinflammation. 2020 Nov 3;17(1):328. doi: 10.1186/s12974-020-02005-x. J Neuroinflammation. 2020. PMID: 33143727 Free PMC article. Review.
-
The contribution of astrocytes and microglia to traumatic brain injury.Br J Pharmacol. 2016 Feb;173(4):692-702. doi: 10.1111/bph.13125. Epub 2015 Apr 24. Br J Pharmacol. 2016. PMID: 25752446 Free PMC article. Review.
Cited by
-
Astrocytes, reactive astrogliosis, and glial scar formation in traumatic brain injury.Neural Regen Res. 2025 Apr 1;20(4):973-989. doi: 10.4103/NRR.NRR-D-23-02091. Epub 2024 May 17. Neural Regen Res. 2025. PMID: 38989932 Free PMC article.
-
Roles of Astrocytic Endothelin ETB Receptor in Traumatic Brain Injury.Cells. 2023 Feb 24;12(5):719. doi: 10.3390/cells12050719. Cells. 2023. PMID: 36899860 Free PMC article. Review.
References
-
- Affandi AJ, Olesek K, Grabowska J, Nijen Twilhaar MK, Rodriguez E, Saris A, Zwart ES, Nossent EJ, Kalay H, de Kok M, Kazemier G, Stockl J, van den Eertwegh AJM, de Gruijl TD, Garcia-Vallejo JJ, Storm G, van Kooyk Y, den Haan JMM (2021) CD169 defines activated CD14(+) monocytes with enhanced CD8(+) T cell activation capacity. Front Immunol 12:697840. 10.3389/fimmu.2021.697840 - PMC - PubMed
-
- Bennett ML, Bennett FC, Liddelow SA, Ajami B, Zamanian JL, Fernhoff NB, Mulinyawe SB, Bohlen CJ, Adil A, Tucker A, Weissman IL, Chang EF, Li G, Grant GA, Hayden Gephart MG, Barres BA (2016) New tools for studying microglia in the mouse and human CNS. Proc Natl Acad Sci U S A 113(12):E1738-1746. 10.1073/pnas.1525528113 - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 81925031/National Natural Science Foundation of China
- 82103775/National Natural Science Foundation of China
- 81972967/National Natural Science Foundation of China
- 81872549/National Natural Science Foundation of China
- 82003389/National Natural Science Foundation of China
- 81820108026/National Natural Science Foundation of China
- 202007030001/Guangzhou Science and Technology Program key projects
- 2020B1212060018/Guangdong Science and Technology Department
- 2019A1515011754/Natural Science Foundation of Guangdong Province
- 2019012/Guangdong-Hong Kong-Macao Greater Bay Area Center for Brain Science and Brain-Inspired Intelligence Fund
- 81801229/Youth Program of National Natural Science Foundation of China
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

