Tilapia prolactin cells are thermosensitive osmoreceptors
- PMID: 35438003
- PMCID: PMC9142157
- DOI: 10.1152/ajpregu.00027.2022
Tilapia prolactin cells are thermosensitive osmoreceptors
Abstract
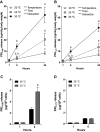
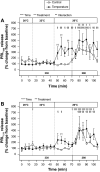
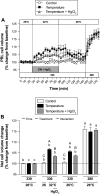
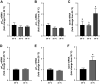
Prolactin (PRL) cells within the rostral pars distalis (RPD) of euryhaline and eurythermal Mozambique tilapia, Oreochromis mossambicus, rapidly respond to a hyposmotic stimulus by releasing two distinct PRL isoforms, PRL188 and PRL177. Here, we describe how environmentally relevant temperature changes affected mRNA levels of prl188 and prl177 and the release of immunoreactive prolactins from RPDs and dispersed PRL cells. When applied under isosmotic conditions (330 mosmol/kgH2O), a 6°C rise in temperature stimulated the release of PRL188 and PRL177 from both RPDs and dispersed PRL cells under perifusion. When exposed to this same change in temperature, ∼50% of dispersed PRL cells gradually increased in volume by ∼8%, a response partially inhibited by the water channel blocker, mercuric chloride. Following their response to increased temperature, PRL cells remained responsive to a hyposmotic stimulus (280 mosmol/kgH2O). The mRNA expression of transient potential vanilloid 4, a Ca2+-channel involved in hyposmotically induced PRL release, was elevated in response to a rise in temperature in dispersed PRL cells and RPDs at 6 and 24 h, respectively; prl188 and prl177 mRNAs were unaffected. Our findings indicate that thermosensitive PRL release is mediated, at least partially, through a cell-volume-dependent pathway similar to how osmoreceptive PRL release is achieved.
Keywords: fish; osmoreceptor; prolactin; thermoreceptor; thermosensitivity.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures

References
-
- Hirano T. The spectrum of prolactin action in teleosts. In: Comparative Endocrinology: Developments and Directions, edited by Ralph CL. New York: A. R. Liss, 1986, p. 53–74. - PubMed
-
- Specker JL, King DS, Nishioka RS, Shirahata K, Yamaguchi K, Bern HA. Isolation and partial characterization of a pair of prolactins released in vitro by the pituitary of cichlid fish, Oreochromis mossambicus. Proc Natl Acad Sci USA 82: 7490–7494, 1985. doi:10.1073/pnas.82.22.7490. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

