Low-Intensity Pulsed Ultrasound Enhances the Efficacy of Bone Marrow-Derived MSCs in Osteoarthritis Cartilage Repair by Regulating Autophagy-Mediated Exosome Release
- PMID: 35438034
- PMCID: PMC9137322
- DOI: 10.1177/19476035221093060
Low-Intensity Pulsed Ultrasound Enhances the Efficacy of Bone Marrow-Derived MSCs in Osteoarthritis Cartilage Repair by Regulating Autophagy-Mediated Exosome Release
Abstract
Objective: The present study explored whether low-intensity pulsed ultrasound (LIPUS) enhances the therapeutic efficacy of mesenchymal stem cells (MSCs) in osteoarthritis (OA) cartilage repair by regulating autophagy-mediated exosome release.
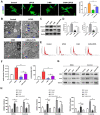
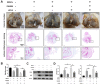
Design: MSCs were isolated from the rat bone marrow and treated with rapamycin, 3-methyladenine, or LIPUS. The mechanism of the LIPUS-stimulated exosome release by MSCs was analyzed by inhibiting autophagy. In addition, the MSCs were co-cultured with OA chondrocytes and stimulated by LIPUS, with or without exosome release inhibitor intervention. The exosome release was detected through transmission electron microscopy (TEM), nanoparticle tracking analysis, and biomarker expression analysis. Autophagy was analyzed through TEM, autophagy-related gene expression analysis, and immunofluorescence analysis in vitro. Furthermore, a rat knee OA model was constructed and treated with MSCs, GW4869, and LIPUS. The cartilage repair was assessed through histopathological analysis and extracellular matrix protein expression analysis.
Results: The in vitro results indicated that LIPUS promoted MSC exosome release by activating autophagy. The in vivo results demonstrated that LIPUS significantly enhanced the positive effects of MSCs on OA cartilage. These effects were significantly blocked by GW4869, an inhibitor of exosome release.
Conclusions: LIPUS can enhance the therapeutic efficacy of MSCs in OA cartilage repair, and the underlying mechanism is related to the increase in autophagy-mediated exosome release.
Keywords: autophagy; exosome; low-intensity pulsed ultrasound; mesenchymal stem cell; osteoarthritis.
Conflict of interest statement
Figures



References
-
- Nelson AE, Allen KD, Golightly YM, Goode AP, Jordan JM. A systematic review of recommendations and guidelines for the management of osteoarthritis: the chronic osteoarthritis management initiative of the U.S. bone and joint initiative. Semin Arthritis Rheum. 2014;43(6):701-12. doi: 10.1016/j.semarthrit.2013.11.012. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

