Two-Dose Severe Acute Respiratory Syndrome Coronavirus 2 Vaccine Effectiveness With Mixed Schedules and Extended Dosing Intervals: Test-Negative Design Studies From British Columbia and Quebec, Canada
- PMID: 35438175
- PMCID: PMC9047203
- DOI: 10.1093/cid/ciac290
Two-Dose Severe Acute Respiratory Syndrome Coronavirus 2 Vaccine Effectiveness With Mixed Schedules and Extended Dosing Intervals: Test-Negative Design Studies From British Columbia and Quebec, Canada
Erratum in
-
Correction to: Two-Dose Severe Acute Respiratory Syndrome Coronavirus 2 Vaccine Effectiveness With Mixed Schedules and Extended Dosing Intervals: Test-Negative Design Studies From British Columbia and Quebec, Canada.Clin Infect Dis. 2023 Feb 18;76(4):778-779. doi: 10.1093/cid/ciac584. Clin Infect Dis. 2023. PMID: 36650055 Free PMC article. No abstract available.
Abstract
Background: The Canadian coronavirus disease 2019 (COVID-19) immunization strategy deferred second doses and allowed mixed schedules. We compared 2-dose vaccine effectiveness (VE) by vaccine type (mRNA and/or ChAdOx1), interval between doses, and time since second dose in 2 of Canada's larger provinces.
Methods: Two-dose VE against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection or hospitalization among adults ≥18 years, including due to Alpha, Gamma, and Delta variants of concern (VOCs), was assessed ≥14 days postvaccination by test-negative design studies separately conducted in British Columbia and Quebec, Canada, between 30 May and 27 November (epi-weeks 22-47) 2021.
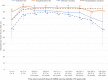
Results: In both provinces, all homologous or heterologous mRNA and/or ChAdOx1 2-dose schedules were associated with ≥90% reduction in SARS-CoV-2 hospitalization risk for ≥7 months. With slight decline from a peak of >90%, VE against infection was ≥80% for ≥6 months following homologous mRNA vaccination, lower by ∼10% when both doses were ChAdOx1 but comparably high following heterologous ChAdOx1 + mRNA receipt. Findings were similar by age group, sex, and VOC. VE was significantly higher with longer 7-8-week versus manufacturer-specified 3-4-week intervals between mRNA doses.
Conclusions: Two doses of any mRNA and/or ChAdOx1 combination gave substantial and sustained protection against SARS-CoV-2 hospitalization, spanning Delta-dominant circulation. ChAdOx1 VE against infection was improved by heterologous mRNA series completion. A 7-8-week interval between first and second doses improved mRNA VE and may be the optimal schedule outside periods of intense epidemic surge. Findings support interchangeability and extended intervals between SARS-CoV-2 vaccine doses, with potential global implications for low-coverage areas and, going forward, for children.
Keywords: SARS-CoV-2; heterologous; test-negative design; vaccine effectiveness; waning.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. G. D. S. received a grant paid to his institution for a meningococcal seroprevalence study from Pfizer in 2016. M. K. received grants/contracts paid to his institution from Roche (related to human papillomavirus), Hologic (related to human papillomavirus), and Siemens (related to human papillomavirus), unrelated to this work. M. S. has been an investigator on projects, unrelated to the current work, funded by GlaxoSmithKline, Merck, Moderna, Pfizer, Sanofi-Pasteur, Seqirus, Symvivo, and VBI Vaccines. All funds have been paid to his institute, and he has not received any personal payments. M. S. is also the Chair/Deputy Chair of 2 Data Safety Monitoring Boards (DSMBs) for coronavirus disease 2019 (COVID-19) vaccine trials, involving different vaccines. R. G. received honoraria for an RSV Coordinators Workshop funded by AbbVie (payment to author). A. N. J. has received funding for other severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) sequencing and COVID-19 vaccine projects, paid to her institution and unrelated to the current work, from Genome BC, the Public Health Agency of Canada and the Canada Foundation for Innovation. D. M. S. reports contracts or grants, paid to her institution and unrelated to the current work, from Michael Smith Foundation for Health Research, Public Health Agency of Canada, and the Canadian Institutes of Health Research. G. D. reports a grant or contract paid to his institution from the Ministère de la Santé et des Services Sociaux du Québec. E. G. reports that their spouse is employed by QHR Tech, an electronic medical records company (no payments to author and author owns no stock in the company). All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures





References
-
- Skowronski DM, De Serres G. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Eng J Med 2021; 384:1576–7. - PubMed
-
- World Health Organization (WHO) . Interim recommendations for use of the Pfizer-BioNTech COVID-19 vaccine, BNT162b2, under Emergency Use Listing. Interim guidance. Geneva, Switzerland: WHO, 2021. Available at:https://assets.documentcloud.org/documents/20445916/who-2019-ncov-vaccin.... Accessed 21 March 2022.
-
- Joint Committee on Vaccination and Immunisation (JCVI) . Advice on priority groups for COVID-19 vaccination. JCVI, 2020. Available at:https://assets.publishing.service.gov.uk/government/uploads/system/uploa.... Accessed 21 March 2022.
-
- Thomas SJ, Moreira ED, Kitchin N, et al. Six month safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. medRxiv [preprint], 28 July 2021. doi: 10.1101/2021.07.28.21261159. - DOI
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

