Palladium-Mediated Incorporation of Carboranes into Small Molecules, Peptides, and Proteins
- PMID: 35438502
- PMCID: PMC9881053
- DOI: 10.1021/jacs.2c01932
Palladium-Mediated Incorporation of Carboranes into Small Molecules, Peptides, and Proteins
Erratum in
-
Correction to "Palladium-Mediated Incorporation of Carboranes into Small Molecules, Peptides, and Proteins".J Am Chem Soc. 2022 Jun 8;144(22):10096. doi: 10.1021/jacs.2c04948. Epub 2022 May 31. J Am Chem Soc. 2022. PMID: 35639117 No abstract available.
Abstract
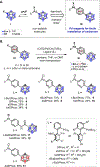
Carboranes represent a class of compounds with increasing therapeutic potential. However, few general approaches to readily embed carboranes into small molecules, peptides, and proteins are available. We report a strategy based on palladium-mediated C-X (X = C, S, and N) bond formation for the installation of carborane-containing moieties onto small molecules and peptides. We demonstrate the ability of Pd-based reagents with appropriate ligands to overcome the high hydrophobicity of the carborane group and enable chemoselective conjugation of cysteine residues at room temperature in aqueous buffer. Accordingly, carboranes can be efficiently installed on proteins by employing a combination of a bis-sulfonated biarylphosphine-ligated Pd reagent in an aqueous histidine buffer. This method is successfully employed on nanobodies, a fully synthetic affibody, and the antibody therapeutics trastuzumab and cetuximab. The conjugates of the affibody ZHER2 and the trastuzumab antibody retained binding to their target antigens. Conjugated proteins maintain their activity in cell-based functional assays in HER2-positive BT-474 cell lines. This approach enables the rapid incorporation of carborane moieties into small molecules, peptides, and proteins for further exploration in boron neutron capture therapy, which requires the targeted delivery of boron-dense groups.
Conflict of interest statement
The authors declare the following competing financial interest(s): B.L.P. is a co-founder and/or member of the scientific advisory board of several companies focusing on the development of protein and peptide therapeutics. The authors filed a provisional patent disclosure regarding the methodology and compounds described in this study. MIT has obtained patents for the palladium ligand described in this work from which S.L.B. and former/current co-workers receive royalty payments.
Figures





References
-
- Stockmann P; Gozzi M; Kuhnert R; Sárosi MB; Hey-Hawkins E New keys for old locks: carborane-containing drugs as platforms for mechanism-based therapies. Chem. Soc. Rev. 2019, 48, 3497–3512. - PubMed
- Scholz M; Hey-Hawkins E Carbaboranes as Pharmacophores: Properties, Synthesis, and Application Strategies. Chem. Rev. 2011, 111, 7035–7062. - PubMed
-
- Kuhnert R; Sárosi M-B; George S; Lönnecke P; Hofmann B; Steinhilber D; Steinmann S; Schneider-Stock R; Murganić B; Mijatović S; Maksimović-Ivanić D; Hey-Hawkins E Carborane-Based Analogues of 5-Lipoxygenase Inhibitors Co-inhibit Heat Shock Protein 90 in HCT116 Cells. ChemMedChem 2019, 14, 255–261. - PubMed
- Qiu Z; Xie Z A Strategy for Selective Catalytic B–H Functionalization of o-Carboranes. Acc. Chem. Res. 2021, 54, 4065–4079. - PubMed
- Quan Y; Xie Z Controlled functionalization of o-carborane via transition metal catalyzed B–H activation. Chem. Soc. Rev. 2019, 48, 3660–3673. - PubMed
-
- Yin Y; Ochi N; Craven TW; Baker D; Takigawa N; Suga H De Novo Carborane-Containing Macrocyclic Peptides Targeting Human Epidermal Growth Factor Receptor. J. Am. Chem. Soc. 2019, 141, 19193–19197. - PubMed
-
- Barth RF; Grecula JC Boron neutron capture therapy at the crossroads - Where do we go from here? Appl. Radiat. Isot. 2020, 160, 109029. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

