CD36, a signaling receptor and fatty acid transporter that regulates immune cell metabolism and fate
- PMID: 35438721
- PMCID: PMC9022290
- DOI: 10.1084/jem.20211314
CD36, a signaling receptor and fatty acid transporter that regulates immune cell metabolism and fate
Abstract
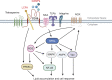
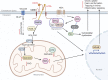
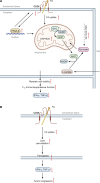
CD36 is a type 2 cell surface scavenger receptor widely expressed in many immune and non-immune cells. It functions as both a signaling receptor responding to DAMPs and PAMPs, as well as a long chain free fatty acid transporter. Recent studies have indicated that CD36 can integrate cell signaling and metabolic pathways through its dual functions and thereby influence immune cell differentiation and activation, and ultimately help determine cell fate. Its expression along with its dual functions in both innate and adaptive immune cells contribute to pathogenesis of common diseases, including atherosclerosis and tumor progression, which makes CD36 and its downstream effectors potential therapeutic targets. This review comprehensively examines the dual functions of CD36 in a variety of immune cells, especially macrophages and T cells. We also briefly discuss CD36 function in non-immune cells, such as adipocytes and platelets, which impact the immune system via intercellular communication. Finally, outstanding questions in this field are provided for potential directions of future studies.
© 2022 Chen et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures
References
-
- Abumrad, N.A., el-Maghrabi M.R., Amri E.Z., Lopez E, and Grimaldi P.A.. 1993. Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36. J. Biol. Chem. 268:17665–17668. - PubMed
-
- Albert, M.L., Pearce S.F., Francisco L.M., Sauter B., Roy P., Silverstein R.L., and Bhardwaj N.. 1998. Immature dendritic cells phagocytose apoptotic cells via alphavbeta5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J. Exp. Med. 188:1359–1368. 10.1084/jem.188.7.1359 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

