Identification of Drug Interaction Adverse Events in Patients With COVID-19: A Systematic Review
- PMID: 35438752
- PMCID: PMC9020212
- DOI: 10.1001/jamanetworkopen.2022.7970
Identification of Drug Interaction Adverse Events in Patients With COVID-19: A Systematic Review
Abstract
Importance: During the COVID-19 pandemic, urgent clinical management of patients has mainly included drugs currently administered for other diseases, referred to as repositioned drugs. As a result, some of these drugs have proved to be not only ineffective but also harmful because of adverse events associated with drug-drug interactions (DDIs).
Objective: To identify DDIs that led to adverse clinical outcomes and/or adverse drug reactions in patients with COVID-19 by systematically reviewing the literature and assessing the value of drug interaction checkers in identifying such events.
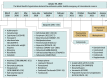
Evidence review: After identification of the drugs used during the COVID-19 pandemic, the drug interaction checkers Drugs.com, COVID-19 Drug Interactions, LexiComp, Medscape, and WebMD were consulted to analyze theoretical DDI-associated adverse events in patients with COVID-19 from March 1, 2020, through February 28, 2022. A systematic literature review was performed by searching the databases PubMed, Scopus, and Cochrane for articles published from March 1, 2020, through February 28, 2022, to retrieve articles describing actual adverse events associated with DDIs. The drug interaction checkers were consulted again to evaluate their potential to assess such events.
Findings: The DDIs identified in the reviewed articles involved 46 different drugs. In total, 575 DDIs for 58 drug pairs (305 associated with at least 1 adverse drug reaction) were reported. The drugs most involved in DDIs were lopinavir and ritonavir. Of the 6917 identified studies, 20 met the inclusion criteria. These studies, which enrolled 1297 patients overall, reported 115 DDI-related adverse events: 15 (26%) were identifiable by all tools analyzed, 29 (50%) were identifiable by at least 1 of them, and 14 (24%) remained nonidentifiable.
Conclusions and relevance: The main finding of this systematic review is that the use of drug interaction checkers could have identified several DDI-associated adverse drug reactions, including severe and life-threatening events. Both the interactions between the drugs used to treat COVID-19 and between the COVID-19 drugs and those already used by the patients should be evaluated.
Conflict of interest statement
Figures
References
-
- Esposito S, Noviello S, Pagliano P. Update on treatment of COVID-19: ongoing studies between promising and disappointing results. Infez Med. 2020;28(2):198-211. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

