Narsoplimab, a Mannan-Binding Lectin-Associated Serine Protease-2 Inhibitor, for the Treatment of Adult Hematopoietic Stem-Cell Transplantation-Associated Thrombotic Microangiopathy
- PMID: 35439028
- PMCID: PMC9467678
- DOI: 10.1200/JCO.21.02389
Narsoplimab, a Mannan-Binding Lectin-Associated Serine Protease-2 Inhibitor, for the Treatment of Adult Hematopoietic Stem-Cell Transplantation-Associated Thrombotic Microangiopathy
Abstract
Purpose: Hematopoietic stem-cell transplantation-associated thrombotic microangiopathy (HSCT-TMA) is a serious complication with significant mortality and no approved therapy. HSCT-TMA results from endothelial injury, which activates the lectin pathway of complement. Narsoplimab (OMS721), an inhibitor of mannan-binding lectin-associated serine protease-2 (MASP-2), was evaluated for safety and efficacy in adults with HSCT-TMA.
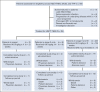
Methods: In this single-arm open-label pivotal trial (NCT02222545), patients received intravenous narsoplimab once weekly for 4-8 weeks. The primary end point (response rate) required clinical improvement in two categories: (1) laboratory TMA markers (both platelet count and lactate dehydrogenase) and (2) organ function or freedom from transfusion. Patients receiving at least one dose (full analysis set [FAS]; N = 28) were analyzed.
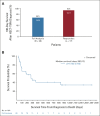
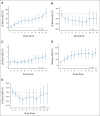
Results: The response rate was 61% in the FAS population. Similar responses were observed across all patient subgroups defined by baseline features, HSCT characteristics, and HSCT complications. Improvement in organ function occurred in 74% of patients in the FAS population. One-hundred-day survival after HSCT-TMA diagnosis was 68% and 94% in FAS population and responders, respectively, whereas median overall survival was 274 days in the FAS population. Narsoplimab was well tolerated, and adverse events were typical of this population, with no apparent safety signal of concern.
Conclusion: In this study, narsoplimab treatment was safe, significantly improved laboratory TMA markers, and resulted in clinical response and favorable overall survival.
Conflict of interest statement
This author is a member of the
No other potential conflicts of interest were reported.
Figures

References
-
- Dietrich S, Falk CS, Benner A, et al. : Endothelial vulnerability and endothelial damage are associated with risk of graft-versus-host disease and response to steroid treatment. Biol Blood Marrow Transplant 19:22-27, 2013 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

