An epilepsy-associated KV1.2 charge-transfer-center mutation impairs KV1.2 and KV1.4 trafficking
- PMID: 35439054
- PMCID: PMC9169947
- DOI: 10.1073/pnas.2113675119
An epilepsy-associated KV1.2 charge-transfer-center mutation impairs KV1.2 and KV1.4 trafficking
Abstract
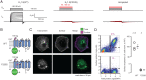
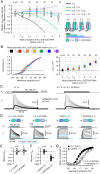
We report on a heterozygous KCNA2 variant in a child with epilepsy. KCNA2 encodes KV1.2 subunits, which form homotetrameric potassium channels and participate in heterotetrameric channel complexes with other KV1-family subunits, regulating neuronal excitability. The mutation causes substitution F233S at the KV1.2 charge transfer center of the voltage-sensing domain. Immunocytochemical trafficking assays showed that KV1.2(F233S) subunits are trafficking deficient and reduce the surface expression of wild-type KV1.2 and KV1.4: a dominant-negative phenotype extending beyond KCNA2, likely profoundly perturbing electrical signaling. Yet some KV1.2(F233S) trafficking was rescued by wild-type KV1.2 and KV1.4 subunits, likely in permissible heterotetrameric stoichiometries: electrophysiological studies utilizing applied transcriptomics and concatemer constructs support that up to one or two KV1.2(F233S) subunits can participate in trafficking-capable heterotetramers with wild-type KV1.2 or KV1.4, respectively, and that both early and late events along the biosynthesis and secretion pathway impair trafficking. These studies suggested that F233S causes a depolarizing shift of ∼48 mV on KV1.2 voltage dependence. Optical tracking of the KV1.2(F233S) voltage-sensing domain (rescued by wild-type KV1.2 or KV1.4) revealed that it operates with modestly perturbed voltage dependence and retains pore coupling, evidenced by off-charge immobilization. The equivalent mutation in the Shaker K+ channel (F290S) was reported to modestly affect trafficking and strongly affect function: an ∼80-mV depolarizing shift, disrupted voltage sensor activation and pore coupling. Our work exposes the multigenic, molecular etiology of a variant associated with epilepsy and reveals that charge-transfer-center disruption has different effects in KV1.2 and Shaker, the archetypes for potassium channel structure and function.
Keywords: channelopathy; dominant negative; fluorometry; ion channel; trafficking.
Conflict of interest statement
The authors declare no competing interest.
Figures






References
-
- Bean B. P., The action potential in mammalian central neurons. Nat. Rev. Neurosci. 8, 451–465 (2007). - PubMed
-
- Kole M. H., Letzkus J. J., Stuart G. J., Axon initial segment Kv1 channels control axonal action potential waveform and synaptic efficacy. Neuron 55, 633–647 (2007). - PubMed
-
- Sheng M., Liao Y. J., Jan Y. N., Jan L. Y., Presynaptic A-current based on heteromultimeric K+ channels detected in vivo. Nature 365, 72–75 (1993). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

