T cell receptor sequencing identifies prior SARS-CoV-2 infection and correlates with neutralizing antibodies and disease severity
- PMID: 35439166
- PMCID: PMC9220924
- DOI: 10.1172/jci.insight.150070
T cell receptor sequencing identifies prior SARS-CoV-2 infection and correlates with neutralizing antibodies and disease severity
Abstract
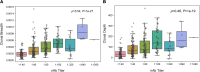
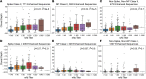
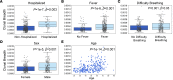
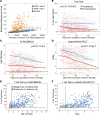
BACKGROUNDMeasuring the immune response to SARS-CoV-2 enables assessment of past infection and protective immunity. SARS-CoV-2 infection induces humoral and T cell responses, but these responses vary with disease severity and individual characteristics.METHODSA T cell receptor (TCR) immunosequencing assay was conducted using small-volume blood samples from 302 individuals recovered from COVID-19. Correlations between the magnitude of the T cell response and neutralizing antibody (nAb) titers or indicators of disease severity were evaluated. Sensitivity of T cell testing was assessed and compared with serologic testing.RESULTSSARS-CoV-2-specific T cell responses were significantly correlated with nAb titers and clinical indicators of disease severity, including hospitalization, fever, and difficulty breathing. Despite modest declines in depth and breadth of T cell responses during convalescence, high sensitivity was observed until at least 6 months after infection, with overall sensitivity ~5% greater than serology tests for identifying prior SARS-CoV-2 infection. Improved performance of T cell testing was most apparent in recovered, nonhospitalized individuals sampled > 150 days after initial illness, suggesting greater sensitivity than serology at later time points and in individuals with less severe disease. T cell testing identified SARS-CoV-2 infection in 68% (55 of 81) of samples with undetectable nAb titers (<1:40) and in 37% (13 of 35) of samples classified as negative by 3 antibody assays.CONCLUSIONThese results support TCR-based testing as a scalable, reliable measure of past SARS-CoV-2 infection with clinical value beyond serology.TRIAL REGISTRATIONSpecimens were accrued under trial NCT04338360 accessible at clinicaltrials.gov.FUNDINGThis work was funded by Adaptive Biotechnologies, Frederick National Laboratory for Cancer Research, NIAID, Fred Hutchinson Joel Meyers Endowment, Fast Grants, and American Society for Transplantation and Cell Therapy.
Keywords: Adaptive immunity; COVID-19; Diagnostics; Infectious disease; T cell receptor.
Figures

Update of
-
T-cell receptor sequencing identifies prior SARS-CoV-2 infection and correlates with neutralizing antibody titers and disease severity.medRxiv [Preprint]. 2021 Mar 22:2021.03.19.21251426. doi: 10.1101/2021.03.19.21251426. medRxiv. 2021. Update in: JCI Insight. 2022 May 23;7(10):e150070. doi: 10.1172/jci.insight.150070. PMID: 33791723 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

