TOP1-DNA Trapping by Exatecan and Combination Therapy with ATR Inhibitor
- PMID: 35439320
- PMCID: PMC9256811
- DOI: 10.1158/1535-7163.MCT-21-1000
TOP1-DNA Trapping by Exatecan and Combination Therapy with ATR Inhibitor
Abstract
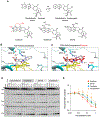
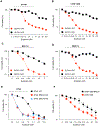
Exatecan and deruxtecan are antineoplastic camptothecin derivatives in development as tumor-targeted-delivery warheads in various formulations including peptides, liposomes, polyethylene glycol nanoparticles, and antibody-drug conjugates. Here, we report the molecular pharmacology of exatecan compared with the clinically approved topoisomerase I (TOP1) inhibitors and preclinical models for validating biomarkers and the combination of exatecan with ataxia telangiectasia and Rad3-related kinase (ATR) inhibitors. Modeling exatecan binding at the interface of a TOP1 cleavage complex suggests two novel molecular interactions with the flanking DNA base and the TOP1 residue N352, in addition to the three known interactions of camptothecins with the TOP1 residues R364, D533, and N722. Accordingly, exatecan showed much stronger TOP1 trapping, higher DNA damage, and apoptotic cell death than the classical TOP1 inhibitors used clinically. We demonstrate the value of SLFN11 expression and homologous recombination (HR) deficiency (HRD) as predictive biomarkers of response to exatecan. We also show that exatecan kills cancer cells synergistically with the clinical ATR inhibitor ceralasertib (AZD6738). To establish the translational potential of this combination, we tested CBX-12, a clinically developed pH-sensitive peptide-exatecan conjugate that selectively targets cancer cells and is currently in clinical trials. The combination of CBX-12 with ceralasertib significantly suppressed tumor growth in mouse xenografts. Collectively, our results demonstrate the potency of exatecan as a TOP1 inhibitor and its clinical potential in combination with ATR inhibitors, using SLFN11 and HRD as predictive biomarkers.
©2022 American Association for Cancer Research.
Conflict of interest statement
Figures





References
-
- Joto N, Ishii M, Minami M, Kuga H, Mitsui I, Tohgo A. DX-8951f, a water-soluble camptothecin analog, exhibits potent antitumor activity against a human lung cancer cell line and its SN-38-resistant variant. Int J Cancer 1997;72(4):680–6 doi 10.1002/(sici)1097-0215(19970807)72:4<680::aid-ijc21>3.0.co;2-e. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

