The role and diagnostic accuracy of serology for COVID-19
- PMID: 35439957
- PMCID: PMC9017961
- DOI: 10.1186/s12879-022-07361-y
The role and diagnostic accuracy of serology for COVID-19
Abstract
Background: The role and performance of various serological tests for the diagnosis of COVID-19 are unclear. This study aimed to evaluate the performance of seven commercially available serological assays for SARS-CoV-2 antibodies by testing COVID-19 cases and controls.
Methods: Adult patients with fever for > 5 days, admitted to a tertiary-care teaching hospital in South India, were enrolled prospectively between June and December 2020. SARS-CoV-2 RT-PCR confirmed patients were classified as cases, and patients with febrile illness with laboratory-confirmed alternative diagnosis and healthy participants were controls. All participants were tested with SCoV-2 Detect™ IgM ELISA kit and SCoV-2 Detect™ IgG ELISA kit (InBios International, Seattle, USA) (Inbios), SARS-CoV-2 Total and SARS-CoV-2 IgG (Siemens Healthcare Diagnostics Inc., Tarrytown, USA) (Siemens), Roche Elecsys® Anti-SARS-CoV-2 (Roche Diagnostics, Rotkreuz, Switzerland) (Roche), Abbott SARS-CoV-2 IgG (Abbott Diagnostics, IL, USA) (Abbott), and Liaison® SARS-CoV-2 S1/S2 IgG (DiaSorinS.p.A., Saluggia, Italy) (Liaison). The sensitivities, specificities, positive predictive values (PPV), negative predictive values (NPV), and accuracies were compared.
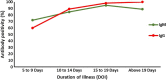
Results: There were 303 participants: 153 cases and 150 controls. ELISA detecting anti-S protein antibody was more sensitive (88.9% for IgG and 86.3% for IgM) than the CLIAs (82.4% for total antibodies and 76.5-85.6% for IgG). Among CLIAs, Roche IgG was most sensitive (85.6%) followed by Abbott (83%) and Liaison (83%). Abbot had the best PPV (88.8%) and was more specific (89.3%) than Liaison (82%) and Roche (82%). Siemens IgG was less sensitive (76.5%) than Siemens Total (82.4%). The specificity of all the serological assays was modest (75-90%). Antibody test positivity increased with the duration of illness reaching 90% after 10 days of illness. When cases were compared against pre-pandemic controls, the IgG gave excellent specificity (98-100%). For seroprevalence studies, InBios IgG had the best accuracy (90.8%) with 88.9% sensitivity and 97.6% specificity.
Conclusion: The serological assays are important adjuncts for the diagnosis of COVID-19 in patients with persistent symptoms, especially in the second week of illness. The value of serological diagnostic tests is limited in the first week of illness and they provide additional value in seroprevalence studies. The diagnostic accuracy of the ELISA and CLIA platforms were comparable.
Keywords: Antibody test; COVID-19; IgG; IgM; SARS-CoV-2; Serology.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
-
- COVID-19 Map [Internet]. Johns Hopkins Coronavirus Resource Center. [cited 2022 Feb]. Available from: https://coronavirus.jhu.edu/map.html.
-
- World Health Organization. (2020). Laboratory testing for coronavirus disease 2019 (COVID-19) in suspected human cases: interim guidance, 2 March 2020. World Health Organization. https://apps.who.int/iris/handle/10665/331329.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous