Meaningful benefits: a framework to assess disease-modifying therapies in preclinical and early Alzheimer's disease
- PMID: 35440022
- PMCID: PMC9017027
- DOI: 10.1186/s13195-022-00984-y
Meaningful benefits: a framework to assess disease-modifying therapies in preclinical and early Alzheimer's disease
Abstract
Background: The need for preventive therapies that interrupt the progression of Alzheimer's disease (AD) before the onset of symptoms or when symptoms are emerging is urgent and has spurred the ongoing development of disease-modifying therapies (DMTs) in preclinical and early AD (mild cognitive impairment [MCI] to mild dementia). Assessing the meaningfulness of what are likely small initial treatment effects in these earlier stages of the AD patho-clinical disease continuum is a major challenge and warrants further consideration. BODY: To accommodate a shift towards earlier intervention in AD, we propose meaningful benefits as a new umbrella concept that encapsulates the spectrum of potentially desirable outcomes that may be demonstrated in clinical trials and other studies across the AD continuum, with an emphasis on preclinical AD and early AD (i.e., MCI due to AD and mild AD dementia). The meaningful benefits framework applies to data collection, assessment, and communication across three dimensions: (1) multidimensional clinical outcome assessments (COAs) including not only core disease outcomes related to cognition and function but also patient- and caregiver-reported outcomes, health and economic outcomes, and neuropsychiatric symptoms; (2) complementary analyses that help contextualize and expand the understanding of COA-based assessments, such as number-needed-to-treat or time-to-event analyses; and (3) assessment of both cumulative benefit and predictive benefit, where early changes on cognitive, functional, or biomarker assessments predict longer-term clinical benefit.
Conclusion: The concept of meaningful benefits emphasizes the importance of multidimensional reporting of clinical trial data while, conceptually, it advances our understanding of treatment effects in preclinical AD and mild cognitive impairment due to AD. We propose that such an approach will help bridge the gap between the emergence of DMTs and their clinical use, particularly now that a DMT is available for patients diagnosed with MCI due to AD and mild AD dementia.
Keywords: Alzheimer’s disease; Biomarkers; Clinical meaningfulness; Clinical trials; Meaningful benefit; Mild cognitive impairment due to Alzheimer’s disease; Preclinical.
© 2022. The Author(s).
Conflict of interest statement
SSA is a full-time employee of Genentech and receives salary and bonuses, and owns company stock.
RAS reports grants from NIH, research funding from Alzheimer’s Association, Janssen, Eli Lilly, and Eisai. She reports receiving personal fees from AC Immune, Acumen, Alnylam, Cytox, Genentech, Janssen, JOMDD, Nervgen, Neuraly, Neurocentria, Oligomerix, Prothena, Renew, Shionogi, and Vigil Neuroscience.
CR has been a paid consultant for several companies developing treatments for Alzheimer’s disease over the last 5 years including Biogen, Eli Lilly, Merck, Roche, Janssen, Abbvie, Kyowa Kirin, Actinogen, and Eisai. He was the UK Chief Investigator for the ENGAGE Trial and Academic Lead on the EPAD (European Prevention of Alzheimer’s Dementia) Programme, which was a public:private partnership between the EU and several companies with an interest in developing treatments for AD
DRK has nothing to disclose.
PSA reports research agreements with Janssen, Lilly and Eisai; grants from NIA, the Alzheimer’s Association, and FNIH and consulting fees from Biogen, Roche, Merck, Abbvie, Immunobrain Checkpoint, Rainbow Medical, and Shionogi.
CL is a full-time employee and shareholder of F. Hoffmann-La Roche Ltd.
AA reports receiving grants to his institution for clinical trials and research from NIA/NIH, Alzheimer’s Clinical Trials Consortium, Alzheimer's Disease Cooperative Study, Alzheimer’s Therapeutics Research Institute, American College of Radiology, Arizona Alzheimer’s Research Consortium, Alzheimer's Prevention Initiative, Biohaven, Global Alzheimer’s Platform, Johns Hopkins, Lilly, NIH/NIA, University of Indiana, University of Southern California, Athira, Alzheon, and Gates Ventures. He reports having received personal fees from AbbVie, Acadia, Allergan, Axovant, AZTherapies, Biogen, Eisai, Grifols, the Japanese Organization for Medical Device Development, Medical Care Corporation, Lundbeck, Merck, Novo Nordisk, Roche/Genentech, Sunovion, Suven, and Synexus. Dr. Atri receives book royalties from Oxford University Press.
JC has provided consultation to AB Science, Acadia, Alkahest, AlphaCognition, ALZPath, Annovis, AriBio, Artery, Avanir, Biogen, Biosplice, Cassava, Cerevel, Clinilabs, Cortexyme, Diadem, EIP Pharma, Eisai, GatehouseBio, GemVax, Genentech, Green Valley, Grifols, Janssen, Karuna, Lexeo, Lilly, Lundbeck, LSP, Merck, NervGen, Novo Nordisk, Oligomerix, Otsuka, PharmacotrophiX, PRODEO, Prothena, ReMYND, Renew, Resverlogix, Roche, Signant Health, Suven, Unlearn AI, Vaxxinity, VigilNeuro, Zai Laboratories pharmaceutical, assessment, and investment companies. Dr Cummings is supported by NIGMS grant P20GM109025; NINDS grant U01NS093334; NIA grant R01AG053798; NIA grant P20AG068053; NIA grant R35AG71476; the Alzheimer’s Disease Drug Discovery Foundation (ADDF); and the Joy Chambers-Grundy Endowment. Dr. Cummings has copyright on the Neuropsychiatric Inventory (NPI).
Figures
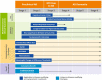

References
-
- Alzheimer’s Association. A public health approach to Alzheimer’s and other dementias. Alzheimer’s Association; 2016. Module 1. https://www.alz.org/media/documents/public-health-approach-alzheimers-fa.... Accessed 15 June 2021.
-
- Carmona R, Elders J, Novello A, Satchher D. U.S. surgeons general: dementia is our top public health crisis. Commentary. Us Against Alzheimer’s. October 10, 2019. https://www.usagainstalzheimers.org/blog/us-surgeons-general-dementia-ou.... Accessed 15 June 2021.
-
- Resolution recognizing Alzheimer’s disease as a public health crisis impacting the nation’s health care infrastructure. National Lieutenant Governors Association; 2018. https://nlga.us/wp-content/uploads/Resolution-Recognizing-Alzheimer%E2%8.... Accessed 15 June 2021.
-
- Alzheimer's disease facts and figures [published online ahead of print, 2020 Mar 10]. Alzheimers Dement. 2020;2020. 10.1002/alz.12068.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

