Intracellular marriage of bicarbonate and Mn ions as "immune ion reactors" to regulate redox homeostasis and enhanced antitumor immune responses
- PMID: 35440088
- PMCID: PMC9020034
- DOI: 10.1186/s12951-022-01404-x
Intracellular marriage of bicarbonate and Mn ions as "immune ion reactors" to regulate redox homeostasis and enhanced antitumor immune responses
Abstract
Background: Different from Fe ions in Fenton reaction, Mn ions can function both as catalyst for chemodynamic therapy and immune adjuvant for antitumor immune responses. In Mn-mediated Fenton-like reaction, bicarbonate ([Formula: see text]), as the most important component to amplify therapeutic effects, must be present, however, intracellular [Formula: see text] is strictly limited because of the tight control by live cells.
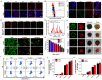
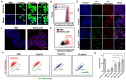
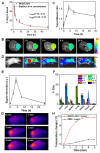
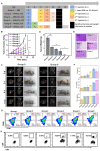
Results: Herein, Stimuli-responsive manganese carbonate-indocyanine green complexes (MnCO3-ICG) were designed for intracellular marriage of bicarbonate and Mn ions as "immune ion reactors" to regulate intracellular redox homeostasis and antitumor immune responses. Under the tumor acidic environment, the biodegradable complex can release "ion reactors" of Mn2+ and [Formula: see text], and ICG in the cytoplasm. The suddenly increased [Formula: see text] in situ inside the cells regulate intracellular pH, and accelerate the generation of hydroxyl radicals for the oxidative stress damage of tumors cells because [Formula: see text] play a critical role to catalyze Mn-mediated Fenton-like reaction. Investigations in vitro and in vivo prove that the both CDT and phototherapy combined with Mn2+-enhanced immunotherapy effectively suppress tumor growth and realize complete tumor elimination.
Conclusions: The combination therapy strategy with the help of novel immune adjuvants would produce an enhanced immune response, and be used for the treatment of deep tumors in situ.
Keywords: Immune activator; Manganese immunotherapy; Orthotopic liver cancer; Redox homeostasis; Self-supplying intracellular ions.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no conflict of interests.
Figures


Similar articles
-
Ion drugs for precise orthotopic tumor management by in situ the generation of toxic ion and drug pools.Theranostics. 2022 Jan 1;12(2):734-746. doi: 10.7150/thno.66468. eCollection 2022. Theranostics. 2022. PMID: 34976210 Free PMC article.
-
Manganese-Based Nanoplatform As Metal Ion-Enhanced ROS Generator for Combined Chemodynamic/Photodynamic Therapy.ACS Appl Mater Interfaces. 2019 Nov 6;11(44):41140-41147. doi: 10.1021/acsami.9b16617. Epub 2019 Oct 23. ACS Appl Mater Interfaces. 2019. PMID: 31603650
-
Near-infrared light-triggered synergistic antitumor therapy based on hollow ZIF-67-derived Co3S4-indocyanine green nanocomplex as a superior reactive oxygen species generator.Mater Sci Eng C Mater Biol Appl. 2021 Nov;130:112465. doi: 10.1016/j.msec.2021.112465. Epub 2021 Sep 30. Mater Sci Eng C Mater Biol Appl. 2021. PMID: 34702540
-
Chemodynamic Therapy via Fenton and Fenton-Like Nanomaterials: Strategies and Recent Advances.Small. 2022 Feb;18(6):e2103868. doi: 10.1002/smll.202103868. Epub 2021 Nov 2. Small. 2022. PMID: 34729913 Review.
-
Fenton/Fenton-like metal-based nanomaterials combine with oxidase for synergistic tumor therapy.J Nanobiotechnology. 2021 Oct 16;19(1):325. doi: 10.1186/s12951-021-01074-1. J Nanobiotechnology. 2021. PMID: 34656118 Free PMC article. Review.
Cited by
-
Tumor microenvironment responsive Mn-based nanoplatform activate cGAS-STING pathway combined with metabolic interference for enhanced anti-tumor therapy.J Nanobiotechnology. 2025 May 25;23(1):377. doi: 10.1186/s12951-025-03453-4. J Nanobiotechnology. 2025. PMID: 40414874 Free PMC article.
-
Dual-modality immune nano-activator harnessing Mn2⁺ and quercetin to potentiate the cGAS-STING pathway for advanced cancer metalloimmunotherapy.J Nanobiotechnology. 2025 Mar 25;23(1):248. doi: 10.1186/s12951-025-03336-8. J Nanobiotechnology. 2025. PMID: 40128784 Free PMC article.
-
Enzyme-catalyzed synthesis of selenium-doped manganese phosphate for synergistic therapy of drug-resistant colorectal cancer.J Nanobiotechnology. 2023 Mar 1;21(1):72. doi: 10.1186/s12951-023-01819-0. J Nanobiotechnology. 2023. PMID: 36859296 Free PMC article.
-
Disrupting calcium homeostasis and glycometabolism in engineered lipid-based pharmaceuticals propel cancer immunogenic death.Acta Pharm Sin B. 2025 Mar;15(3):1255-1267. doi: 10.1016/j.apsb.2024.12.018. Epub 2024 Dec 21. Acta Pharm Sin B. 2025. PMID: 40370551 Free PMC article.
-
Ca & Mn dual-ion hybrid nanostimulator boosting anti-tumor immunity via ferroptosis and innate immunity awakening.Bioact Mater. 2023 Dec 3;33:483-496. doi: 10.1016/j.bioactmat.2023.11.017. eCollection 2024 Mar. Bioact Mater. 2023. PMID: 38125638 Free PMC article.
References
-
- Liu J, Chen Q, Feng L, Liu Z. Nanomedicine for tumor microenvironment modulation and cancer treatment enhancement. Nano Today. 2018;21:55–73. doi: 10.1016/j.nantod.2018.06.008. - DOI
MeSH terms
Substances
Grants and funding
- 82001956/National Natural Science Foundation of China
- 82172007, 81771977/National Natural Science Foundation of China
- BX20200196/the National Postdoctoral Program for Innovative Talents
- 2021J06007/the Science Fund for Distinguished Young Scholars of Fujian Province
- 3502Z20183017/the Xiamen Science and Technology Plan Project
LinkOut - more resources
Full Text Sources
Medical

