Impact of APOE genotype on prion-type propagation of tauopathy
- PMID: 35440098
- PMCID: PMC9019935
- DOI: 10.1186/s40478-022-01359-y
Impact of APOE genotype on prion-type propagation of tauopathy
Abstract
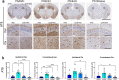
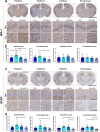
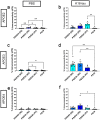
Apolipoprotein (APOE) is a major risk factor of Alzheimer's disease (AD), with the E2, E3 and E4 isoforms differentially regulating the burden of AD-associated neuropathologies, such as amyloid β and tau. In AD, pathological tau is thought to spread along neuroanatomic connections following a prion-like mechanism. To provide insights into whether APOE isoforms differentially regulate the prion properties of tau and determine trans-synaptic transmission of tauopathy, we have generated human P301S mutant tau transgenic mice (PS19) that carry human APOE (APOE2, APOE3 or APOE4) or mouse Apoe allele. Mice received intrahippocamal injections of preformed aggregates of K18-tau at young ages, which were analyzed 5 months post-inoculation. Compared to the parental PS19 mice with mouse Apoe alleles, PS19 mice expressing human APOE alleles generally responded to K18-tau seeding with more intense AT8 immunoreactive phosphorylated tau athology. APOE3 homozygous mice accumulated higher levels of AT8-reactive ptau and microgliosis relative to APOE2 or APOE4 homozygotes (E3 > E4~2). PS19 mice that were heterozygous for APOE3 showed similar results, albeit to a lesser degree. In the timeframe of our investigation, we did not observe significant induction of argentophilic or MC1-reactive neurofibrillary tau tangle in PS19 mice homozygous for human APOE. To our knowledge, this is the first comprehensive study in rodent models that provides neuropathological insights into the dose-dependent effect of APOE isoforms on phosphorylated tau pathology induced by recombinant tau prions.
Keywords: APOE; Phosphorylated tau; Prion; Tangle.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests exist.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

