Sintilimab versus placebo in combination with chemotherapy as first line treatment for locally advanced or metastatic oesophageal squamous cell carcinoma (ORIENT-15): multicentre, randomised, double blind, phase 3 trial
- PMID: 35440464
- PMCID: PMC9016493
- DOI: 10.1136/bmj-2021-068714
Sintilimab versus placebo in combination with chemotherapy as first line treatment for locally advanced or metastatic oesophageal squamous cell carcinoma (ORIENT-15): multicentre, randomised, double blind, phase 3 trial
Abstract
Objective: To evaluate sintilimab versus placebo in combination with chemotherapy (cisplatin plus paclitaxel or cisplatin plus 5-fluorouracil) as first line treatment of unresectable locally advanced, recurrent, or metastatic oesophageal squamous cell carcinoma.
Design: Multicentre, randomised, double blind, phase 3 trial.
Setting: 66 sites in China and 13 sites outside of China between 14 December 2018 and 9 April 2021.
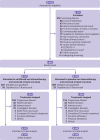
Participants: 659 adults (aged ≥18 years) with advanced or metastatic oesophageal squamous cell carcinoma who had not received systemic treatment.
Intervention: Participants were randomised 1:1 to receive sintilimab or placebo (3 mg/kg in patients weighing <60 kg or 200 mg in patients weighing ≥60 kg) in combination with cisplatin 75 mg/m2 plus paclitaxel 175 mg/m2 every three weeks. The trial was amended to allow investigators to choose the chemotherapy regimen: cisplatin plus paclitaxel or cisplatin plus 5-fluorouracil (800 mg/m2 continuous infusion on days 1-5).
Main outcome measures: Overall survival in all patients and in patients with combined positive scores of ≥10 for expression of programmed cell death ligand 1.
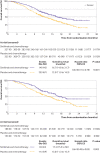
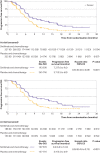
Results: 659 patients were randomly assigned to sintilimab (n=327) or placebo (n=332) with chemotherapy. 616 of 659 patients (93%) received sintilimab or placebo in combination with cisplatin plus paclitaxel and 43 of 659 patients (7%) received sintilimab or placebo in combination with cisplatin plus 5-fluorouracil. At the interim analysis, sintilimab with chemotherapy showed better overall survival compared with placebo and chemotherapy in all patients (median 16.7 v 12.5 months, hazard ratio 0.63, 95% confidence interval 0.51 to 0.78, P<0.001) and in patients with combined positive scores of ≥10 (17.2 v 13.6 months, 0.64, 0.48 to 0.85, P=0.002). Sintilimab and chemotherapy significantly improved progression free survival compared with placebo and chemotherapy in all patients (7.2 v 5.7 months, 0.56, 0.46 to 0.68, P<0.001) and in patients with combined positive scores of ≥10 (8.3 v 6.4 months, 0.58, 0.45 to 0.75, P<0.001). Adverse events related to treatment occurred in 321 of 327 patients (98%) in the sintilimab-chemotherapy group versus 326 of 332 (98%) patients in the placebo-chemotherapy group. Rates of adverse events related to treatment, grade ≥3, were 60% (196/327) and 55% (181/332) in the sintilimab-chemotherapy and placebo-chemotherapy groups, respectively.
Conclusions: Compared with placebo, sintilimab in combination with cisplatin plus paclitaxel showed significant benefits in overall survival and progression free survival as first line treatment in patients with advanced or metastatic oesophageal squamous cell carcinoma. Similar benefits of sintilimab with cisplatin plus 5-fluorouracil seem promising.
Trial registration: ClinicalTrials.gov NCT03748134.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support from Innovent Biologics and Eli Lilly for the submitted work; LS reports receiving research funding from Innovent Biologics, Beijing Xiantong Biomedical Technology, Qilu Pharmaceutical, ZaiLab Pharmaceutical, Beihai Kangcheng (Beijing) Medical Technology, and Jacobio Pharmaceuticals; consultant fees from MSD, Merck, Mingji Biopharmaceutical, Haichuang Pharmaceutical, Herbour Biomed, and BI; honoraria from Hutchison Whampoa, Hengrui, ZaiLab, and CSTONE pharmaceutical; and serving as a consultant for Rongchang Pharmaceutical, ZaiLab, CSTONE Pharmaceutical, and BMS. AZ has participated in consulting boards, advisory boards, or both, for Amgen, Lilly, Merck, Roche, Sanofi, Servier, Baxter, MSD, Pierre Fabre, Havas Life, Alira Health, and Zymeworks.
Figures


Comment in
-
Chemoimmunotherapy in advanced esophageal squamous cell carcinoma: optimizing chemotherapy regimens for immunotherapy combinations.Signal Transduct Target Ther. 2022 Jul 13;7(1):233. doi: 10.1038/s41392-022-01077-w. Signal Transduct Target Ther. 2022. PMID: 35831276 Free PMC article. No abstract available.
References
-
- GBD 2017 Oesophageal Cancer Collaborators . The global, regional, and national burden of oesophageal cancer and its attributable risk factors in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol 2020;5:582-97. 10.1016/S2468-1253(20)30007-8 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
