Chronic Cortical Inflammation, Cognitive Impairment, and Immune Reactivity Associated with Diffuse Brain Injury Are Ameliorated by Forced Turnover of Microglia
- PMID: 35440489
- PMCID: PMC9121837
- DOI: 10.1523/JNEUROSCI.1910-21.2022
Chronic Cortical Inflammation, Cognitive Impairment, and Immune Reactivity Associated with Diffuse Brain Injury Are Ameliorated by Forced Turnover of Microglia
Abstract
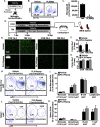
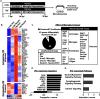
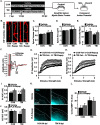
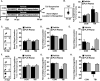
Traumatic brain injury (TBI) is associated with an increased risk of cognitive, psychiatric, and neurodegenerative complications that may develop after injury. Increased microglial reactivity following TBI may underlie chronic neuroinflammation, neuropathology, and exaggerated responses to immune challenges. Therefore, the goal of this study was to force turnover of trauma-associated microglia that develop after diffuse TBI and determine whether this alleviated chronic inflammation, improved functional recovery and attenuated reduced immune reactivity to lipopolysaccharide (LPS) challenge. Male mice received a midline fluid percussion injury (mFPI) and 7 d later were subjected to a forced microglia turnover paradigm using CSF1R antagonism (PLX5622). At 30 d postinjury (dpi), cortical gene expression, dendritic complexity, myelin content, neuronal connectivity, cognition, and immune reactivity were assessed. Myriad neuropathology-related genes were increased 30 dpi in the cortex, and 90% of these gene changes were reversed by microglial turnover. Reduced neuronal connectivity was evident 30 dpi and these deficits were attenuated by microglial turnover. TBI-associated dendritic remodeling and myelin alterations, however, remained 30 dpi independent of microglial turnover. In assessments of functional recovery, increased depressive-like behavior, and cognitive impairment 30 dpi were ameliorated by microglia turnover. To investigate microglial priming and reactivity 30 dpi, mice were injected intraperitoneally with LPS. This immune challenge caused prolonged lethargy, sickness behavior, and microglial reactivity in the TBI mice. These extended complications with LPS in TBI mice were prevented by microglia turnover. Collectively, microglial turnover 7 dpi alleviated behavioral and cognitive impairments associated with microglial priming and immune reactivity 30 dpi.SIGNIFICANCE STATEMENT A striking feature of traumatic brain injury (TBI), even mild injuries, is that over 70% of individuals have long-term neuropsychiatric complications. Chronic inflammatory processes are implicated in the pathology of these complications and these issues can be exaggerated by immune challenge. Therefore, our goal was to force the turnover of microglia 7 d after TBI. This subacute 7 d postinjury (dpi) time point is a critical transitional period in the shift toward chronic inflammatory processes and microglia priming. This forced microglia turnover intervention in mice attenuated the deficits in behavior and cognition 30 dpi. Moreover, microglia priming and immune reactivity after TBI were also reduced with microglia turnover. Therefore, microglia represent therapeutic targets after TBI to reduce persistent neuroinflammation and improve recovery.
Keywords: brain injury; cognition; forced turnover; inflammation; lipopolysaccharide; microglia.
Copyright © 2022 the authors.
Figures

References
-
- Bachstetter AD, Van Eldik LJ, Schmitt FA, Neltner JH, Ighodaro ET, Webster SJ, Patel E, Abner EL, Kryscio RJ, Nelson PT (2015) Disease-related microglia heterogeneity in the hippocampus of Alzheimer's disease, dementia with Lewy bodies, and hippocampal sclerosis of aging. Acta Neuropathol Commun 3:32. 10.1186/s40478-015-0209-z - DOI - PMC - PubMed
-
- Barrett JP, Henry RJ, Shirey KA, Doran SJ, Makarevich OD, Ritzel RM, Meadows VA, Vogel SN, Faden AI, Stoica BA, Loane DJ (2020) Interferon-beta plays a detrimental role in experimental traumatic brain injury by enhancing neuroinflammation that drives chronic neurodegeneration. J Neurosci 40:2357–2370. 10.1523/JNEUROSCI.2516-19.2020 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
