Albuminuria-Lowering Effect of Dapagliflozin, Eplerenone, and Their Combination in Patients with Chronic Kidney Disease: A Randomized Crossover Clinical Trial
- PMID: 35440501
- PMCID: PMC9342643
- DOI: 10.1681/ASN.2022020207
Albuminuria-Lowering Effect of Dapagliflozin, Eplerenone, and Their Combination in Patients with Chronic Kidney Disease: A Randomized Crossover Clinical Trial
Abstract
Background: Sodium glucose cotransporter 2 (SGLT2) inhibitors and mineralocorticoid receptor antagonists (MRAs) reduce the urinary albumin-to-creatinine ratio (UACR) and confer kidney and cardiovascular protection in patients with CKD. We assessed efficacy and safety of the SGLT2 inhibitor dapagliflozin and MRA eplerenone alone and in combination in patients with CKD.
Methods: We conducted a randomized open-label crossover trial in patients with urinary albumin excretion ≥100 mg/24 hr, eGFR 30-90 ml/min per 1.73 m2, who had been receiving maximum tolerated stable doses of an ACE inhibitor (ACEi) or angiotensin receptor blocker (ARB). Patients were assigned to 4-week treatment periods with dapagliflozin 10 mg/day, eplerenone 50 mg/day, or their combination in random order, separated by 4-week washout periods. Primary outcome was the correlation in UACR changes between treatments. Secondary outcome was the percent change in 24-hour UACR from baseline.
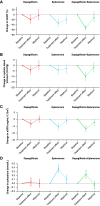
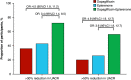
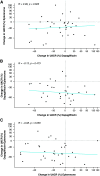
Results: Of 57 patients screened, 46 were randomly assigned (mean eGFR, 58.1 ml/min per 1.73 m2; median UACR, 401 mg/g) to the three groups. Mean percentage change from baseline in UACR after 4 weeks of treatment with dapagliflozin, eplerenone, and dapagliflozin-eplerenone was -19.6% (95% confidence interval [CI], -34.3 to -1.5), -33.7% (95% CI, -46.1 to -18.5), and -53% (95% CI, -61.7 to -42.4; P<0.001 versus dapagliflozin; P=0.01 versus eplerenone). UACR change during dapagliflozin or eplerenone treatment did not correlate with UACR change during dapagliflozin-eplerenone (r=-0.13; P=0.47; r=-0.08; P=0.66, respectively). Hyperkalemia was more frequently reported with eplerenone (n=8; 17.4%) compared with dapagliflozin (n=0; 0%) or dapagliflozin-eplerenone (n=2; 4.3%; P between-groups=0.003).
Conclusions: Albuminuria changes in response to dapagliflozin and eplerenone did not correlate, supporting systematic rotation of these therapies to optimize treatment. Combining dapagliflozin with eplerenone resulted in a robust additive UACR-lowering effect. A larger trial in this population is required to confirm long-term efficacy and safety of combined SGLT2 inhibitor and MRA treatment.
Clinical trial registry name and registration number: European Union Clinical Trials Register, EU 2017-004641-25.
Keywords: albuminuria; aldosterone; chronic kidney disease; dapagliflozin; eplerenone; mineralocorticoid receptor antagonist; randomized controlled trials; sodium glucose co transporter.
Copyright © 2022 by the American Society of Nephrology.
Figures


References
-
- Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. ; CREDENCE Trial Investigators : Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 380: 2295–2306, 2019 - PubMed
-
- Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, et al. ; DAPA-CKD Trial Committees and Investigators : Dapagliflozin in patients with chronic kidney disease. N Engl J Med 383: 1436–1446, 2020 - PubMed
-
- Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Rossing P, et al. ; FIDELIO-DKD Investigators : Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med 383: 2219–2229, 2020 - PubMed
-
- Pitt B, Filippatos G, Agarwal R, Anker SD, Bakris GL, Rossing P, et al. ; FIGARO-DKD Investigators : Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med 385: 2252–2263, 2021 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

