Distinct and additive effects of calorie restriction and rapamycin in aging skeletal muscle
- PMID: 35440545
- PMCID: PMC9018781
- DOI: 10.1038/s41467-022-29714-6
Distinct and additive effects of calorie restriction and rapamycin in aging skeletal muscle
Erratum in
-
Author Correction: Distinct and additive effects of calorie restriction and rapamycin in aging skeletal muscle.Nat Commun. 2022 Apr 27;13(1):2410. doi: 10.1038/s41467-022-30189-8. Nat Commun. 2022. PMID: 35477974 Free PMC article. No abstract available.
Abstract
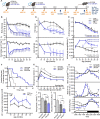
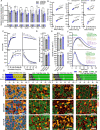
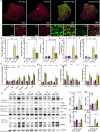
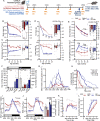
Preserving skeletal muscle function is essential to maintain life quality at high age. Calorie restriction (CR) potently extends health and lifespan, but is largely unachievable in humans, making "CR mimetics" of great interest. CR targets nutrient-sensing pathways centering on mTORC1. The mTORC1 inhibitor, rapamycin, is considered a potential CR mimetic and is proven to counteract age-related muscle loss. Therefore, we tested whether rapamycin acts via similar mechanisms as CR to slow muscle aging. Here we show that long-term CR and rapamycin unexpectedly display distinct gene expression profiles in geriatric mouse skeletal muscle, despite both benefiting aging muscles. Furthermore, CR improves muscle integrity in mice with nutrient-insensitive, sustained muscle mTORC1 activity and rapamycin provides additive benefits to CR in naturally aging mouse muscles. We conclude that rapamycin and CR exert distinct, compounding effects in aging skeletal muscle, thus opening the possibility of parallel interventions to counteract muscle aging.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

