TNF is a potential therapeutic target to suppress prostatic inflammation and hyperplasia in autoimmune disease
- PMID: 35440548
- PMCID: PMC9018703
- DOI: 10.1038/s41467-022-29719-1
TNF is a potential therapeutic target to suppress prostatic inflammation and hyperplasia in autoimmune disease
Abstract
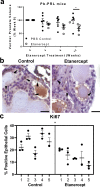
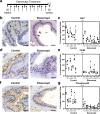
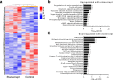
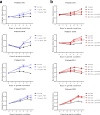
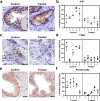
Autoimmune (AI) diseases can affect many organs; however, the prostate has not been considered to be a primary target of these systemic inflammatory processes. Here, we utilize medical record data, patient samples, and in vivo models to evaluate the impact of inflammation, as seen in AI diseases, on prostate tissue. Human and mouse tissues are used to examine whether systemic targeting of inflammation limits prostatic inflammation and hyperplasia. Evaluation of 112,152 medical records indicates that benign prostatic hyperplasia (BPH) prevalence is significantly higher among patients with AI diseases. Furthermore, treating these patients with tumor necrosis factor (TNF)-antagonists significantly decreases BPH incidence. Single-cell RNA-seq and in vitro assays suggest that macrophage-derived TNF stimulates BPH-derived fibroblast proliferation. TNF blockade significantly reduces epithelial hyperplasia, NFκB activation, and macrophage-mediated inflammation within prostate tissues. Together, these studies show that patients with AI diseases have a heightened susceptibility to BPH and that reducing inflammation with a therapeutic agent can suppress BPH.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Comment in
-
Could TNF-antagonists be a novel treatment strategy for BPH patients?Cell Stress. 2022 Jun 7;6(6):65-67. doi: 10.15698/cst2022.06.268. eCollection 2022 Jun. Cell Stress. 2022. PMID: 36072128 Free PMC article.
-
Uro-Science.J Urol. 2022 Dec;208(6):1340-1342. doi: 10.1097/JU.0000000000002980. Epub 2022 Sep 26. J Urol. 2022. PMID: 36154669 No abstract available.
References
-
- McVary KT. BPH: epidemiology and comorbidities. Am. J. Manag Care. 2006;12:S122–S128. - PubMed
-
- Fowke JH, Koyama T, Fadare O, Clark PE. Does inflammation mediate the obesity and bph relationship? An epidemiologic analysis of body composition and inflammatory markers in blood, urine, and prostate tissue, and the relationship with prostate enlargement and lower urinary tract symptoms. PLoS ONE. 2016;11:e0156918. doi: 10.1371/journal.pone.0156918. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

