Microridge-like structures anchor motile cilia
- PMID: 35440631
- PMCID: PMC9018822
- DOI: 10.1038/s41467-022-29741-3
Microridge-like structures anchor motile cilia
Erratum in
-
Author Correction: Microridge-like structures anchor motile cilia.Nat Commun. 2024 Sep 23;15(1):8252. doi: 10.1038/s41467-024-52323-4. Nat Commun. 2024. PMID: 39313506 Free PMC article. No abstract available.
Abstract
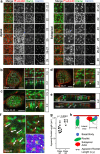
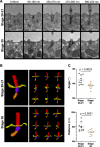
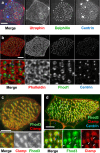
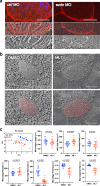
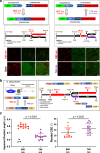
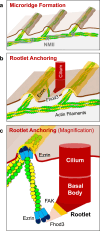
Several tissues contain cells with multiple motile cilia that generate a fluid or particle flow to support development and organ functions; defective motility causes human disease. Developmental cues orient motile cilia, but how cilia are locked into their final position to maintain a directional flow is not understood. Here we find that the actin cytoskeleton is highly dynamic during early development of multiciliated cells (MCCs). While apical actin bundles become increasingly more static, subapical actin filaments are nucleated from the distal tip of ciliary rootlets. Anchorage of these subapical actin filaments requires the presence of microridge-like structures formed during MCC development, and the activity of Nonmuscle Myosin II. Optogenetic manipulation of Ezrin, a core component of the microridge actin-anchoring complex, or inhibition of Myosin Light Chain Kinase interfere with rootlet anchorage and orientation. These observations identify microridge-like structures as an essential component of basal body rootlet anchoring in MCCs.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Hirokawa, N., Tanaka, Y., Okada, Y. & Takeda, S. Nodal flow and the generation of left-right asymmetry. Cell125, 33–45 (2006). - PubMed
-
- Mitchell, B., Jacobs, R., Li, J., Chien, S. & Kintner, C. A positive feedback mechanism governs the polarity and motion of motile cilia. Nature447, 97–101 (2007). - PubMed
-
- Wallmeier, J. et al. Motile ciliopathies. Nat. Rev. Dis. Prim.6, 77 (2020). - PubMed
-
- Singla, V. & Reiter, J. F. The primary cilium as the cell’s antenna: signaling at a sensory organelle. Science313, 629–633 (2006). - PubMed
-
- Nigg, E. A. & Raff, J. W. Centrioles, centrosomes, and cilia in health and disease. Cell139, 663–678 (2009). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

