Identification and characterization of novel endolysins targeting Gardnerella vaginalis biofilms to treat bacterial vaginosis
- PMID: 35440653
- PMCID: PMC9018826
- DOI: 10.1038/s41522-022-00285-0
Identification and characterization of novel endolysins targeting Gardnerella vaginalis biofilms to treat bacterial vaginosis
Abstract
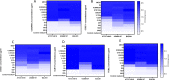
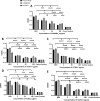
Bacterial vaginosis (BV) is a recurrent dysbiosis that is frequently associated with preterm birth, increased risk for acquisition of human immunodeficiency virus (HIV) and other sexually transmitted infections (STIs). The overgrowth of a key pathobiont, Gardnerella vaginalis, as a recalcitrant biofilm is central to the development of this dysbiosis. Overgrowth of vaginal biofilms, seeded by initial G. vaginalis colonization, leads to recurrent symptomatic BV which is poorly resolved by classically used antibiotics. In this light, the use of bacteriophages and/or their proteins, represents a promising alternative. Here we identify 84 diverse anti-Gardnerella endolysins across 7 protein families. A subset of 36 endolysin candidates were refactored and overexpressed in an E. coli BL21 (DE3) system and 5 biochemically and structurally diverse endolysins were fully characterized. Each candidate endolysin showed good lytic activity against planktonic G. vaginalis ATCC14018, as well as G. vaginalis clinical isolates. These endolysin candidates were assayed in biofilm prevention and disruption assays, with biofilm disruption at low microgram concentrations (5 μg/ml) observed. In addition to clonal G. vaginalis biofilms, endolysin candidates could also successfully disrupt polyspecies biofilms. Importantly, none of our candidates showed lytic activity against commensal lactobacilli present in the vaginal microbiota such as L. crispatus, L. jensenii, L. gasseri, and L. iners or against Atopobium vaginae (currently classified as Fannyhessa vaginae). The potency and selectivity of these novel endolysins constitute a promising alternative treatment to combat BV, avoiding problems associated with antibiotic resistance, while retaining beneficial commensal bacteria in the vaginal flora. The diverse library of candidates reported here represents a strong repository of endolysins for further preclinical development.
© 2022. The Author(s).
Conflict of interest statement
M.C. and D.B.C. of CC Biotech Ltd have a patent for the clinical use of the endolysins described in this study. The remaining authors declare no competing interests.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

