Evidence for postnatal neurogenesis in the human amygdala
- PMID: 35440676
- PMCID: PMC9018740
- DOI: 10.1038/s42003-022-03299-8
Evidence for postnatal neurogenesis in the human amygdala
Abstract
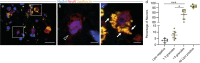
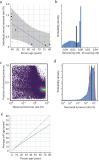
The human amygdala is involved in processing of memory, decision-making, and emotional responses. Previous studies suggested that the amygdala may represent a neurogenic niche in mammals. By combining two distinct methodological approaches, lipofuscin quantification and 14C-based retrospective birth dating of neurons, along with mathematical modelling, we here explored whether postnatal neurogenesis exists in the human amygdala. We investigated post-mortem samples of twelve neurologically healthy subjects. The average rate of lipofuscin-negative neurons was 3.4%, representing a substantial proportion of cells substantially younger than the individual. Mass spectrometry analysis of genomic 14C-concentrations in amygdala neurons compared with atmospheric 14C-levels provided evidence for postnatal neuronal exchange. Mathematical modelling identified a best-fitting scenario comprising of a quiescent and a renewing neuronal population with an overall renewal rate of >2.7% per year. In conclusion, we provide evidence for postnatal neurogenesis in the human amygdala with cell turnover rates comparable to the hippocampus.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

