Engineered cellular immunotherapies in cancer and beyond
- PMID: 35440724
- PMCID: PMC9305718
- DOI: 10.1038/s41591-022-01765-8
Engineered cellular immunotherapies in cancer and beyond
Abstract
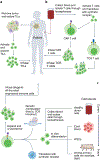
This year marks the tenth anniversary of cell therapy with chimeric antigen receptor (CAR)-modified T cells for refractory leukemia. The widespread commercial approval of genetically engineered T cells for a variety of blood cancers offers hope for patients with other types of cancer, and the convergence of human genome engineering and cell therapy technology holds great potential for generation of a new class of cellular therapeutics. In this Review, we discuss the goals of cellular immunotherapy in cancer, key challenges facing the field and exciting strategies that are emerging to overcome these obstacles. Finally, we outline how developments in the cancer field are paving the way for cellular immunotherapeutics in other diseases.
© 2022. Springer Nature America, Inc.
Figures



References
-
- Combination Products (FDA, accessed 1 March 2022); https://www.fda.gov/combination-products
-
- Frangoul H et al. CRISPR–Cas9 gene editing for sickle cell disease and β-thalassemia. N. Engl. J. Med 384, 252–260 (2021). - PubMed
-
- Approved Cellular and Gene Therapy (FDA, 2022); https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-produ...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

