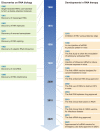
RNA therapy: rich history, various applications and unlimited future prospects
- PMID: 35440755
- PMCID: PMC9016686
- DOI: 10.1038/s12276-022-00757-5
RNA therapy: rich history, various applications and unlimited future prospects
Abstract
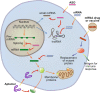
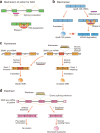
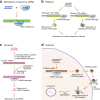
RNA therapy refers to the treatment or prevention of diseases using RNA-based molecules. The recent advent of a series of effective messenger RNA-based vaccines in response to the COVID-19 pandemic has reignited research interest in RNA therapy. Based on the accumulated results of long-term research in the field of RNA therapy spanning several decades, therapeutic agents for various diseases are being rapidly developed. These therapeutics tend to target diseases that cannot be treated with other conventional drug groups, and several clinical studies are underway for a variety of RNA-based therapeutics against various incurable diseases. This review describes the history of several important discoveries in RNA biology and their impact on key developments in RNA therapy as well as the advantages of RNA therapy. In addition, it describes the action mechanisms and examples of drugs approved for RNA therapy. Finally, this review discusses methods for RNA drug delivery to target organs and cells. Given that RNA therapy is expected to advance and play an integral role in the development of novel therapeutic agents for human diseases in the future, this review is designed to offer an updated reference point for researchers in this field.
© 2022. The Author(s).
Conflict of interest statement
The author declares no competing interests.
Figures
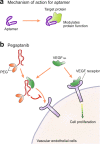
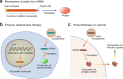
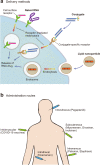
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

