Using a mechanistic framework to model the density of an aquatic parasite Ceratonova shasta
- PMID: 35441056
- PMCID: PMC9013479
- DOI: 10.7717/peerj.13183
Using a mechanistic framework to model the density of an aquatic parasite Ceratonova shasta
Abstract
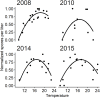
Ceratonova shasta is a myxozoan parasite endemic to the Pacific Northwest of North America that is linked to low survival rates of juvenile salmonids in some watersheds such as the Klamath River basin. The density of C. shasta actinospores in the water column is typically highest in the spring (March-June), and directly influences infection rates for outmigrating juvenile salmonids. Current management approaches require quantities of C. shasta density to assess disease risk and estimate survival of juvenile salmonids. Therefore, we developed a model to simulate the density of waterborne C. shasta actinospores using a mechanistic framework based on abiotic drivers and informed by empirical data. The model quantified factors that describe the key features of parasite abundance during the period of juvenile salmon outmigration, including the week of initial detection (onset), seasonal pattern of spore density, and peak density of C. shasta. Spore onset was simulated by a bio-physical degree-day model using the timing of adult salmon spawning and accumulation of thermal units for parasite development. Normalized spore density was simulated by a quadratic regression model based on a parabolic thermal response with river water temperature. Peak spore density was simulated based on retained explanatory variables in a generalized linear model that included the prevalence of infection in hatchery-origin Chinook juveniles the previous year and the occurrence of flushing flows (≥171 m3/s). The final model performed well, closely matched the initial detections (onset) of spores, and explained inter-annual variations for most water years. Our C. shasta model has direct applications as a management tool to assess the impact of proposed flow regimes on the parasite, and it can be used for projecting the effects of alternative water management scenarios on disease-induced mortality of juvenile salmonids such as with an altered water temperature regime or with dam removal.
Keywords: Actinospore; Disease; Klamath River; Management; Myxozoan; Predictive model; Salmonids.
©2022 Robinson et al.
Conflict of interest statement
The authors declare there are no competing interests.
Figures





References
-
- Alexander JD, Hallett SL, Stocking RW, Xue L, Bartholomew JL. Host and parasite populations after a ten year flood: Manayunkia speciosa and Ceratonova (syn Ceratomyxa) shasta in the Klamath River. Northwest Science. 2014;88(3):219–233. doi: 10.3955/046.088.0305. - DOI
-
- Alexander JD, Kerans BL, El-Matbouli M, Hallett SL, Stevens L. Myxozoan evolution, ecology and development. Springer; Cham: 2015. Annelid-myxosporean interactions; pp. 217–234.
-
- Alexander JD, Wright KA, Som NA, Hetrick NJ, Bartholomew JL. Integrating models to predict distribution of the invertebrate host of myxosporean parasites. Freshwater Science. 2016;35:1263–1275. doi: 10.1086/688342. - DOI
-
- Atkinson SD, Bartholomew JL, Rouse GW. The invertebrate host of salmonid fish parasites Ceratonova shasta and Parvicapsula minibicornis (Cnidaria: Myxozoa), is a novel fabriciid annelid, Manayunkia occidentalis sp. nov. (Sabellida: Fabriciidae) Zootaxa. 2020;4751(2):310–320. doi: 10.11646/zootaxa.4751.2.6. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

